Nuvail by EPI Health, Inc NUVAIL- liquid
Nuvail by
Drug Labeling and Warnings
Nuvail by is a Other medication manufactured, distributed, or labeled by EPI Health, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
INTENDED USE
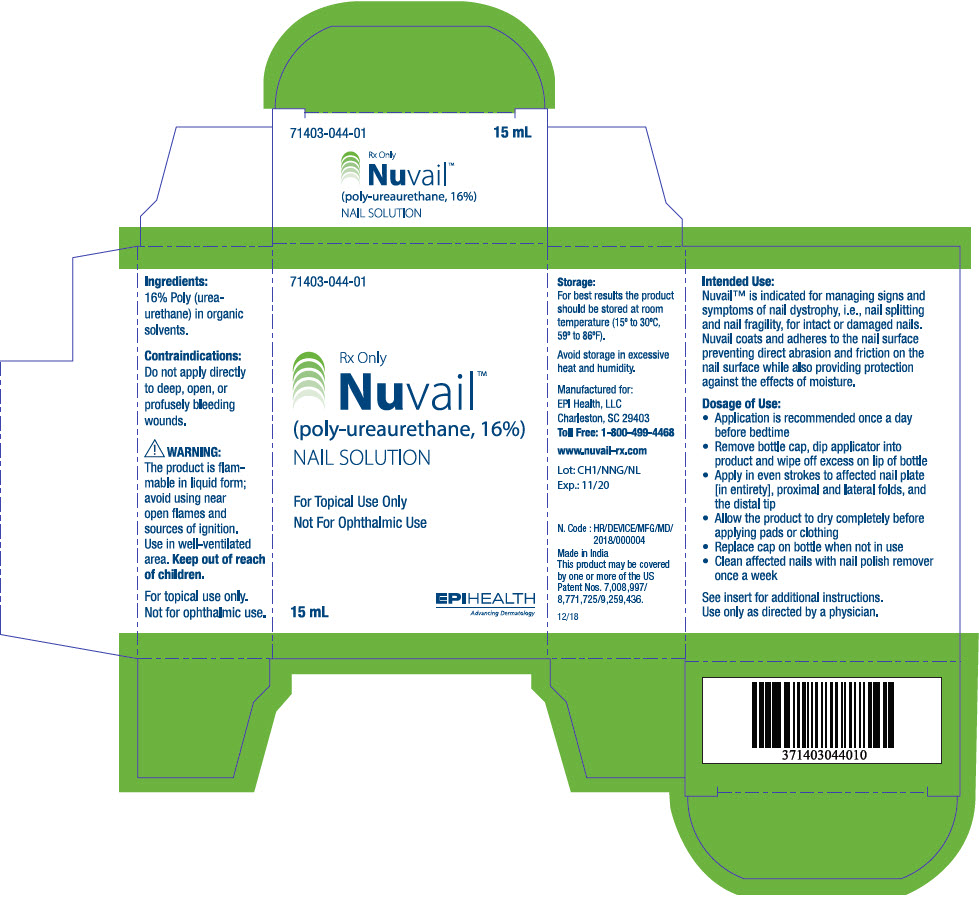
Nuvail™ is indicated for managing signs and symptoms of nail dystrophy, i.e., nail splitting and nail fragility, for intact or damaged nails. Nuvail coats and adheres to the nail surface preventing direct abrasion and friction on the nail surface while also providing protection against the effects of moisture.
-
PRODUCT DESCRIPTION
The product is a biocompatible, polymeric solution which forms a uniform film when applied to the nail. The product is dispersed in a non-cytotoxic solution which dries rapidly, adhering to the contours of the nail to form a flexible, waterproof barrier. The film will wear off naturally and must be re-applied as directed to be effective. The film is colorless, transparent and possesses good moisture vapor permeability.
- INGREDIENTS
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
The product is flammable in liquid form; avoid using near open flames and sources of ignition. Use in well-ventilated area. Keep out of reach of children.
Store at room temperature away from heat. Do not allow product to come into contact with floors, counter tops, furniture or other finished surfaces - will stain.
May temporarily sting upon application. Persons sensitized to isocyanate should not use this product. Should redness or other signs of irritation appear, discontinue use and consult your healthcare provider.
Use of other products, ointments, creams or lotions before application of this product may prevent the film from forming correctly and reduce effectiveness.
-
DIRECTIONS FOR USE
- Application is recommended once a day before bedtime
- Remove bottle cap, dip applicator into product and wipe off excess on lip of bottle
- Apply in even strokes to affected nail plate [in entirety], proximal and lateral folds, and the distal tip
- Allow the product to dry completely before applying pads or clothing
- Replace cap with applicator on bottle when not in use
- Clean affected nails with nail polish remover once a week
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
NUVAIL
bandage, liquid liquidProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:71403-044 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LAURIC ACID (UNII: 1160N9NU9U) (LAURIC ACID - UNII:1160N9NU9U) LAURIC ACID 2.4 mg in 15 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71403-044-01 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date PREMARKET NOTIFICATION K120059 05/01/2017 Labeler - EPI Health, Inc (080638894)
Trademark Results [Nuvail]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NUVAIL 76711716 4499848 Live/Registered |
EPI HEALTH, LLC 2012-06-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.