HYDROMORPHONE HYDROCHLORIDE suppository
HYDROMORPHONE HYDROCHLORIDE by
Drug Labeling and Warnings
HYDROMORPHONE HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Paddock Laboratories, LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING:
HYDROMORPHONE HYDROCHLORIDE SUPPOSITORIES CONTAIN HYDROMORPHONE, WHICH IS A POTENT SCHEDULE II CONTROLLED OPIOID AGONIST. SCHEDULE II OPIOID AGONISTS, INCLUDING MORPHINE, OXYMORPHONE, FENTANYL, AND METHADONE, HAVE THE HIGHEST POTENTIAL FOR ABUSE AND RISK OF PRODUCING RESPIRATORY DEPRESSION. ALCOHOL, OTHER OPIOIDS AND CENTRAL NERVOUS SYSTEM DEPRESSANTS (SEDATIVE-HYPNOTICS) POTENTIATE THE RESPIRATORY DEPRESSANT EFFECTS OF HYDROMORPHONE, INCREASING THE RISK OF RESPIRATORY DEPRESSION THAT MIGHT RESULT IN DEATH.
-
DESCRIPTION
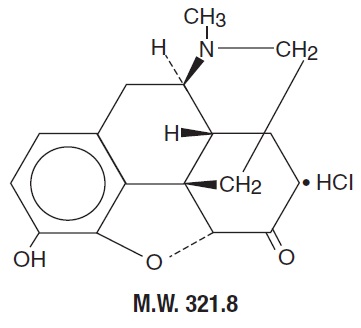
Hydromorphone hydrochloride (WARNING: May be habit forming), a hydrogenated ketone of morphine, is a opioid analgesic. It is available in:
Suppositories (for rectal administration) containing:
3 mg hydromorphone hydrochloride in a cocoa butter base with silicon dioxide.
The structural formula of hydromorphone hydrochloride is:
-
CLINICAL PHARMACOLOGY
Hydromorphone hydrochloride is a opioid analgesic; its principal therapeutic effect is relief of pain. The precise mechanism of action of hydromorphone hydrochloride and other opiates is not known, although it is believed to relate to the existence of opiate receptors in the central nervous system. There is no intrinsic limit to the analgesic effect of hydromorphone hydrochloride; like morphine, adequate doses will relieve even the most severe pain. Clinically, however, dosage limitations are imposed by the adverse effects, primarily respiratory depression, nausea, and vomiting, which can result from high doses.
Hydromorphone hydrochloride has diverse additional actions. It may produce drowsiness, changes in mood and mental clouding, depress the respiratory center and the cough center, stimulate the vomiting center, produce pinpoint constriction of the pupil, enhance parasympathetic activity, elevate cerebrospinal fluid pressure, increase biliary pressure, produce transient hyperglycemia.
Generally, the analgesic action of parenterally administered hydromorphone hydrochloride is apparent within 15 minutes and usually remains in effect for more than five hours. The onset of action of oral hydromorphone hydrochloride is somewhat slower, with measurable analgesia occurring within 30 minutes.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Hydromorphone hydrochloride is contraindicated in patients with a known hypersensitivity to hydromorphone; in the presence of an intracranial lesion associated with increased intracranial pressure; and whenever ventilatory function is depressed (chronic obstructive pulmonary disease, cor pulmonale, emphysema, kyphoscoliosis, status asthmaticus).
-
WARNINGS
Respiratory Depression:
Hydromorphone hydrochloride produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone hydrochloride also affects centers that control respiratory rhythm, and may produce irregular and periodic breathing.
Head Injury and Increased Intracranial Pressure:
The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a preexisting increase in intracranial pressure. Furthermore, opioids produce effects which may obscure the clinical course of patients with head injuries.
-
PRECAUTIONS
Special Risk Patients:
Hydromorphone hydrochloride should be used with caution in elderly or debilitated patients and those with impaired renal or hepatic function, hypothyroidism, Addison’s disease, prostatic hypertrophy or urethral stricture. As with any opioid analgesic agent, the usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Cough Reflex:
Hydromorphone hydrochloride suppresses the cough reflex; as with all opioids, caution should be exercised when hydromorphone hydrochloride is used postoperatively and in patients with pulmonary disease.
Usage in Ambulatory Patients:
Opioids may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery; patients should be cautioned accordingly.
Drug Interactions:
Patients receiving other opioid analgesics, general anesthetics, phenothiazines, tranquilizers, sedative-hypnotics, tricyclic antidepressants or other CNS depressants (including alcohol) concomitantly with hydromorphone hydrochloride may exhibit an additive CNS depression. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Pregnancy:
Pregnancy Category C.
Hydromorphone hydrochloride has been shown to be teratogenic in hamsters when given in doses 600 times the human dose. There are no adequate and well-controlled studies in pregnant women. Hydromorphone hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects:
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Chlorpromazine 0.7 to 1.0 mg/kg q6h, phenobarbital 2 mg/kg q6h, and paregoric 2 to 4 drops/kg q4h, have been used to treat withdrawal symptoms in infants. The duration of therapy is 4 to 28 days, with the dosages decreased as tolerated.
Labor and Delivery:
As with all opioids, administration of hydromorphone hydrochloride to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydromorphone hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
Central Nervous System:
Sedation, drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, dizziness, psychic dependence, mood changes.
Gastrointestinal System:
Nausea, and vomiting occur infrequently; they are more frequent in ambulatory than in recumbent patients. The antiemetic phenothiazines are useful in suppressing these effects; however, some phenothiazine derivatives seem to be antianalgesic and to increase the amount of opioid required to produce pain relief, while other phenothiazines reduce the amount of opioid required to produce a given level of analgesia. Prolonged administration of hydromorphone hydrochloride may produce constipation. Opiate agonist-induced increase in intraluminal pressure may endanger surgical anastomosis.
Cardiovascular System:
Circulatory depression, peripheral circulatory collapse and cardiac arrest have occurred after rapid intravenous injection. Orthostatic hypotension and fainting may occur if a patient stands up suddenly after receiving an injection of hydromorphone hydrochloride.
Genitourinary System:
Ureteral spasm, spasm of vesical sphincters and urinary retention have been reported.
Respiratory Depression:
Hydromorphone hydrochloride produces dose-related respiratory depression by acting directly on brain stem respiratory centers. Hydromorphone hydrochloride also affects centers that control respiratory rhythm, and may produce irregular and periodic breathing. If significant respiratory depression occurs, it may be antagonized by the use of naloxone hydrochloride. The usual adult dose of 0.4 to 0.8 mg given intramuscularly or intravenously, promptly reverses the effects of morphine-like opioid agonists such as hydromorphone hydrochloride. In patients who are physically dependent, small doses of naloxone may be sufficient not only to antagonize respiratory depression, but also to precipitate withdrawal phenomena. The dose of naloxone should therefore be adjusted accordingly in such patients. Since the duration of action of hydromorphone hydrochloride may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. Apply other supportive measures when indicated.
-
DRUG ABUSE AND DEPENDENCE
Hydromorphone hydrochloride is a Schedule II opioid. Psychic dependence, physical dependence, and tolerance may develop upon repeated administration of opioids; therefore, hydromorphone hydrochloride should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when hydromorphone hydrochloride is used for a short time for the treatment of pain. Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, usually assumes clinically significant proportions only after several weeks of continued opioid use, although some mild degree of physical dependence may develop after a few days of opioid therapy. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients.
-
OVERDOSAGE
Signs and Symptoms:
Serious overdosage with hydromorphone hydrochloride is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, particularly the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Treatment:
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. The opioid antagonist naloxone hydrochloride is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to opioids, including hydromorphone hydrochloride. Therefore, naloxone hydrochloride should be administered as described under Adverse Reactions (see Respiratory Depression) in conjunction with ventilatory assistance.
Since the duration of action of hydromorphone hydrochloride may exceed that of the antagonist, the patient should be kept under continued surveillance; repeated doses of the antagonist may be required to maintain adequate respiration. An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Rectal Suppositories: 3 mg Suppositories
Boxes of 6 NDC: 0574-7224-06
Hydromorphone Hydrochloride Suppositories should be stored in a refrigerator. Protect from light.
A Schedule CII Opioid
DEA Order Form Required
Rx Only
Manufactured By
Perrigo®
Minneapolis, MN 55427
2124145 7C000 RC J1 Rev 09-15 B
-
PRINCIPAL DISPLAY PANEL - 3 mg
Rx Only
NDC 0574-7224-06
HYDROmorphone Hydrochloride 3 mg Suppositories
CII
Warning: May be habit forming
UNIT DOSE
FOR RECTAL USE ONLY
6 Suppositories
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
HYDROMORPHONE HYDROCHLORIDE
hydromorphone hydrochloride suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0574-7224 Route of Administration RECTAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROMORPHONE HYDROCHLORIDE (UNII: L960UP2KRW) (HYDROMORPHONE - UNII:Q812464R06) HYDROMORPHONE HYDROCHLORIDE 3 mg Inactive Ingredients Ingredient Name Strength COCOA BUTTER (UNII: 512OYT1CRR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0574-7224-06 6 in 1 BOX 01/31/1996 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/31/1996 Labeler - Paddock Laboratories, LLC (967694121)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.