PROPAFENONE HYDROCHLORIDE capsule, extended release
Propafenone Hydrochloride by
Drug Labeling and Warnings
Propafenone Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PROPAFENONE HYDROCHLORIDE EXTENDED-RELEASE CAPSULES safely and effectively. See full prescribing information for PROPAFENONE HYDROCHLORIDE EXTENDED-RELEASE CAPSULES.
PROPAFENONE HYDROCHLORIDE extended-release capsules, for oral use
Initial U.S. Approval: 1989WARNING: MORTALITY
See full prescribing information for complete boxed warning.
- An increased rate of death or reversed cardiac arrest rate was seen in patients treated with encainide or flecainide (Class IC antiarrhythmics) compared with that seen in patients assigned to placebo. At present, it is prudent to consider any IC antiarrhythmic to have a significant risk of provoking proarrhythmic events in patients with structural heart disease.
- Given the lack of any evidence that these drugs improve survival, antiarrhythmic agents should generally be avoided in patients with nonlife-threatening ventricular arrhythmias, even if the patients are experiencing unpleasant, but not life-threatening, symptoms or signs.
INDICATIONS AND USAGE
Propafenone hydrochloride extended-release capsules are an antiarrhythmic indicated to prolong the time to recurrence of symptomatic atrial fibrillation (AF) in patients with episodic (most likely paroxysmal or persistent) AF who do not have structural heart disease. (1)
Usage Considerations:
- Use in patients with permanent atrial fibrillation or with atrial flutter or paroxysmal supraventricular tachycardia (PSVT) has not been evaluated. Do not use to control ventricular rate during atrial fibrillation. (1)
- In patients with atrial fibrillation and atrial flutter, use propafenone hydrochloride extended-release capsules with drugs that increase the atrioventricular nodal refractory period. (1)
- The effect of propafenone on mortality has not been determined. (1)
DOSAGE AND ADMINISTRATION
- Initiate therapy with 225 mg given every 12 hours. (2)
- Dosage may be increased at a minimum of 5-day intervals to 325 mg every 12 hours and, if necessary, to 425 mg every 12 hours. (2)
- Consider reducing the dose in patients with hepatic impairment, significant widening of the QRS complex, or second- or third-degree AV block. (2)
DOSAGE FORMS AND STRENGTHS
Capsules: 225 mg, 325 mg, 425 mg. (3)
CONTRAINDICATIONS
- Heart failure (4)
- Cardiogenic shock (4)
- Sinoatrial, atrioventricular, and intraventricular disorders of impulse generation and/or conduction in the absence of pacemaker (4)
- Known Brugada Syndrome (4)
- Bradycardia (4)
- Marked hypotension (4)
- Bronchospastic disorders and severe obstructive pulmonary disease (4)
- Marked electrolyte imbalance (4)
WARNINGS AND PRECAUTIONS
- May cause new or worsened arrhythmias. Evaluate patients via ECG prior to and during therapy. (5.1)
- Propafenone hydrochloride extended-release capsules may unmask Brugada or Brugada-like Syndrome. Evaluate patients via ECG after initiation of therapy. (4, 5.2)
- Avoid use with other antiarrhythmic agents or drugs that prolong the QT interval. (5.3)
- Avoid simultaneous use of propafenone with both a cytochrome P450 2D6 (CYP2D6) inhibitor and a 3A4 inhibitor (CYP3A4). (5.4)
- May provoke overt heart failure. (5.5)
- May cause dose-related first-degree AV block or other conduction disturbances. Should not be given to patients with conduction defects in absence of a pacemaker. (5.6)
- May affect artificial pacemakers. Pacemakers should be monitored during therapy. (5.7)
- Agranulocytosis: Patients should report signs of infection. (5.8)
- Administer cautiously to patients with impaired hepatic and renal function. (5.9, 5.10)
- Exacerbation of myasthenia gravis has been reported. (5.11)
ADVERSE REACTIONS
The most commonly reported adverse events with propafenone (greater than 5% and greater than placebo) excluding those not reasonably associated with the use of the drug included the following: dizziness, palpitations, chest pain, dyspnea, taste disturbance, nausea, fatigue, anxiety, constipation, upper respiratory tract infection, edema, and influenza. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Inhibitors of CYP2D6, 1A2, and 3A4 may increase propafenone levels which may lead to cardiac arrhythmias. Simultaneous use with both a CYP3A4 and CYP2D6 inhibitor (or in patients with CYP2D6 deficiency) should be avoided. (7.1)
- Propafenone may increase digoxin or warfarin levels. (7.2, 7.3)
- Orlistat may reduce propafenone concentrations. Abrupt cessation of orlistat in patients stable on propafenone hydrochloride extended-release capsules has resulted in convulsions, atrioventricular block, and circulatory failure. (7.4)
- Concomitant use of lidocaine may increase central nervous system side effects. (7.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Proarrhythmic Effects
5.2 Unmasking Brugada Syndrome
5.3 Use with Drugs that Prolong the QT Interval and Antiarrhythmic Agents
5.4 Drug Interactions: Simultaneous Use with Inhibitors of Cytochrome P450 Isoenzymes 2D6 and 3A4
5.5 Use in Patients with a History of Heart Failure
5.6 Conduction Disturbances
5.7 Effects on Pacemaker Threshold
5.8 Agranulocytosis
5.9 Use in Patients with Hepatic Dysfunction
5.10 Use in Patients with Renal Dysfunction
5.11 Use in Patients with Myasthenia Gravis
5.12 Elevated ANA Titers
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 CYP2D6 and CYP3A4 Inhibitors
7.2 Digoxin
7.3 Warfarin
7.4 Orlistat
7.5 Beta-Antagonists
7.6 Lidocaine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MORTALITY
- In the National Heart, Lung, and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicenter, randomized, double-blind trial in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than 6 days but less than 2 years previously, an increased rate of death or reversed cardiac arrest rate (7.7%; 56/730) was seen in patients treated with encainide or flecainide (Class IC antiarrhythmics) compared with that seen in patients assigned to placebo (3.0%; 22/725). The average duration of treatment with encainide or flecainide in this trial was 10 months.
- The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) or other antiarrhythmic drugs is uncertain, but at present, it is prudent to consider any IC antiarrhythmic to have a significant proarrhythmic risk in patients with structural heart disease. Given the lack of any evidence that these drugs improve survival, antiarrhythmic agents should generally be avoided in patients with non-life-threatening ventricular arrhythmias, even if the patients are experiencing unpleasant, but not life-threatening, symptoms or signs.
-
1 INDICATIONS AND USAGE
Propafenone hydrochloride extended-release capsules are indicated to prolong the time to recurrence of symptomatic atrial fibrillation (AF) in patients with episodic (most likely paroxysmal or persistent) AF who do not have structural heart disease.
Usage Considerations:
- The use of propafenone hydrochloride extended-release capsules in patients with permanent AF or in patients exclusively with atrial flutter or paroxysmal supraventricular tachycardia (PSVT) has not been evaluated. Do not use propafenone hydrochloride extended-release capsules to control ventricular rate during AF.
- Some patients with atrial flutter treated with propafenone have developed 1:1 conduction, producing an increase in ventricular rate. Concomitant treatment with drugs that increase the functional atrioventricular (AV) nodal refractory period is recommended.
- The effect of propafenone on mortality has not been determined [see Boxed Warning].
-
2 DOSAGE AND ADMINISTRATION
Propafenone hydrochloride extended-release capsules can be taken with or without food. Do not crush or further divide the contents of the capsule.
The dose of propafenone hydrochloride extended-release capsules must be individually titrated on the basis of response and tolerance. Initiate therapy with propafenone hydrochloride extended-release capsules 225 mg given every 12 hours. Dosage may be increased at a minimum of 5-day intervals to 325 mg given every 12 hours. If additional therapeutic effect is needed, the dose of propafenone hydrochloride extended-release capsules may be increased to 425 mg given every 12 hours.
In patients with hepatic impairment or those with significant widening of the QRS complex or second- or third-degree AV block, consider reducing the dose.
The combination of cytochrome P450 3A4 (CYP3A4) inhibition and either cytochrome P450 2D6 (CYP2D6) deficiency or CYP2D6 inhibition with the simultaneous administration of propafenone may significantly increase the concentration of propafenone and thereby increase the risk of proarrhythmia and other adverse events. Therefore, avoid simultaneous use of propafenone hydrochloride extended-release capsules with both a CYP2D6 inhibitor and a CYP3A4 inhibitor [see Warnings and Precautions (5.4), Drug Interactions (7.1)].
-
3 DOSAGE FORMS AND STRENGTHS
Propafenone Hydrochloride Extended-Release Capsules, USP 225 mg are white opaque cap/ white opaque body, size ‘1’ hard gelatin capsule shells, imprinted with “PRF 225” on cap in circular orientation with red ink.
Propafenone Hydrochloride Extended-Release Capsules, USP 325 mg are white opaque cap/ white opaque body, size ‘0’ hard gelatin capsule shells, imprinted with “PRF 325” on cap in circular orientation and single band around the circumference on the body with red ink.
Propafenone Hydrochloride Extended-Release Capsules, USP 425 mg are white opaque cap/ white opaque body, size ‘00’ hard gelatin capsule shells, imprinted with “PRF 425” on cap in circular orientation and triple band around the circumference on the body with red ink.
-
4 CONTRAINDICATIONS
Propafenone hydrochloride extended-release capsules are contraindicated in the following circumstances:
- Heart failure
- Cardiogenic shock
- Sinoatrial, atrioventricular, and intraventricular disorders of impulse generation or conduction (e.g., sick sinus node syndrome, AV block) in the absence of an artificial pacemaker
- Known Brugada Syndrome
- Bradycardia
- Marked hypotension
- Bronchospastic disorders or severe obstructive pulmonary disease
- Marked electrolyte imbalance
-
5 WARNINGS AND PRECAUTIONS
5.1 Proarrhythmic Effects
Propafenone has caused new or worsened arrhythmias. Such proarrhythmic effects include sudden death and life-threatening ventricular arrhythmias such as ventricular fibrillation, ventricular tachycardia, asystole, and torsade de pointes. It may also worsen premature ventricular contractions or supraventricular arrhythmias, and it may prolong the QT interval. It is therefore essential that each patient given propafenone hydrochloride extended-release capsules be evaluated electrocardiographically prior to and during therapy to determine whether the response to propafenone hydrochloride extended-release capsules supports continued treatment. Because propafenone prolongs the QRS interval in the electrocardiogram, changes in the QT interval are difficult to interpret [see Clinical Pharmacology (12.2)].
In the Propafenone Hydrochloride Extended-Release Capsules Atrial Fibrillation Trial (RAFT) trial [see Clinical Studies (14)], there were too few deaths to assess the long-term risk to patients. There were 5 deaths, 3 in the pooled group for propafenone hydrochloride extended-release capsules (0.8%), and 2 in the placebo group (1.6%). In the overall database of 8 trials of propafenone hydrochloride extended-release capsules and immediate-release propafenone hydrochloride, the mortality rate was 2.5% per year on propafenone and 4.0% per year on placebo. Concurrent use of propafenone with other antiarrhythmic agents has not been well studied.
In a U.S. uncontrolled, open-label, multicenter trial using the immediate-release formulation in patients with symptomatic supraventricular tachycardia (SVT), 1.9% (9/474) of these patients experienced ventricular tachycardia (VT) or ventricular fibrillation (VF) during the trial. However, in 4 of the 9 patients, the ventricular tachycardia was of atrial origin. Six of the 9 patients that developed ventricular arrhythmias did so within 14 days of onset of therapy. About 2.3% (11/474) of all patients had recurrence of SVT during the trial which could have been a change in the patients’ arrhythmia behavior or could represent a proarrhythmic event. Case reports in patients treated with propafenone for atrial fibrillation/flutter have included increased premature ventricular contractions (PVCs), VT, VF, torsades de pointes, asystole, and death.
Overall in clinical trials with propafenone hydrochloride immediate-release (which included patients treated for ventricular arrhythmias, atrial fibrillation/flutter, and PSVT), 4.7% of all patients had new or worsened ventricular arrhythmia possibly representing a proarrhythmic event (0.7% was an increase in PVCs; 4.0% a worsening or new appearance of VT or VF). Of the patients who had worsening of VT (4%), 92% had a history of VT and/or VT/VF, 71% had coronary artery disease, and 68% had a prior myocardial infarction. The incidence of proarrhythmia in patients with less serious or benign arrhythmias, which include patients with an increase in frequency of PVCs, was 1.6%. Although most proarrhythmic events occurred during the first week of therapy, late events also were seen and the CAST trial [see Boxed Warning: Mortality] suggests that an increased risk of proarrythmia is present throughout treatment.
5.2 Unmasking Brugada Syndrome
Brugada Syndrome may be unmasked after exposure to propafenone hydrochloride extended-release capsules. Perform an ECG after initiation of propafenone hydrochloride extended-release capsules and discontinue the drug if changes are suggestive of Brugada Syndrome [see Contraindications (4)].
5.3 Use with Drugs that Prolong the QT Interval and Antiarrhythmic Agents
The use of propafenone hydrochloride extended-release capsules in conjunction with other drugs that prolong the QT interval has not been extensively studied. Such drugs may include many antiarrhythmics, some phenothiazines, tricyclic antidepressants, and oral macrolides. Withhold Class IA and III antiarrhythmic agents for at least 5 half-lives prior to dosing with propafenone hydrochloride extended-release capsules. Avoid the use of propafenone with Class IA and III antiarrhythmic agents (including quinidine and amiodarone). There is only limited experience with the concomitant use of Class IB or IC antiarrhythmics.
5.4 Drug Interactions: Simultaneous Use with Inhibitors of Cytochrome P450 Isoenzymes 2D6 and 3A4
Propafenone is metabolized by CYP2D6, CYP3A4, and CYP1A2 isoenzymes. Approximately 6% of Caucasians in the U.S. population are naturally deficient in CYP2D6 activity and other demographic groups are deficient to a somewhat lesser extent. Drugs that inhibit these CYP pathways (such as desipramine, paroxetine, ritonavir, sertraline for CYP2D6; ketoconazole, erythromycin, saquinavir, and grapefruit juice for CYP3A4; and amiodarone and tobacco smoke for CYP1A2) can be expected to cause increased plasma levels of propafenone.
Increased exposure to propafenone may lead to cardiac arrhythmias and exaggerated beta-adrenergic blocking activity. Because of its metabolism, the combination of CYP3A4 inhibition and either CYP2D6 deficiency or CYP2D6 inhibition in users of propafenone is potentially hazardous. Therefore, avoid simultaneous use of propafenone hydrochloride extended-release capsules with both a CYP2D6 inhibitor and a CYP3A4 inhibitor.
5.5 Use in Patients with a History of Heart Failure
Propafenone exerts a negative inotropic activity on the myocardium as well as beta-blockade effects and may provoke overt heart failure. In the U.S. trial (RAFT) in patients with symptomatic AF, heart failure was reported in 4 (1.0%) patients receiving propafenone hydrochloride extended-release capsules (all doses) compared with 1 (0.8%) patient receiving placebo. Proarrhythmic effects more likely occur when propafenone is administered to patients with heart failure (NYHA III and IV) or severe myocardial ischemia [see Contraindications (4)].
In clinical trial experience with propafenone hydrochloride immediate-release, new or worsened congestive heart failure has been reported in 3.7% of patients with ventricular arrhythmia. These events were more likely in subjects with pre-existing heart failure and coronary artery disease. New onset of heart failure attributable to propafenone developed in less than 0.2% of patients with ventricular arrhythmia and in 1.9% of patients with paroxysmal AF or PSVT.
5.6 Conduction Disturbances
Propafenone slows atrioventricular conduction and may also cause dose-related first-degree AV block. Average PR interval prolongation and increases in QRS duration are also dose-related. Do not give propafenone to patients with atrioventricular and intraventricular conduction defects in the absence of a pacemaker [see Contraindications (4), Clinical Pharmacology (12.2)].
In a U.S. trial (RAFT) in 523 patients with a history of symptomatic AF treated with propafenone hydrochloride extended-release capsules, sinus bradycardia (rate less than 50 beats/min) was reported with the same frequency with propafenone hydrochloride extended-release capsules and placebo.
5.7 Effects on Pacemaker Threshold
Propafenone may alter both pacing and sensing thresholds of implanted pacemakers and defibrillators. During and after therapy, monitor and re-program these devices accordingly.
5.8 Agranulocytosis
Agranulocytosis has been reported in patients receiving propafenone. Generally, the agranulocytosis occurred within the first 2 months of propafenone therapy, and upon discontinuation of therapy the white count usually normalized by 14 days. Unexplained fever or decrease in white cell count, particularly during the initial 3 months of therapy, warrant consideration of possible agranulocytosis or granulocytopenia. Instruct patients to report promptly any signs of infection such as fever, sore throat, or chills.
5.9 Use in Patients with Hepatic Dysfunction
Propafenone is highly metabolized by the liver. Severe liver dysfunction increases the bioavailability of propafenone to approximately 70% compared with 3% to 40% in patients with normal liver function when given propafenone hydrochloride immediate-release tablets. In 8 patients with moderate to severe liver disease administered propafenone hydrochloride immediate-release tablets, the mean half-life was approximately 9 hours. No trials have compared bioavailability of propafenone from propafenone hydrochloride extended-release capsules in patients with normal and impaired hepatic function. Increased bioavailability of propafenone in these patients may result in excessive accumulation. Carefully monitor patients with impaired hepatic function for excessive pharmacological effects [see Overdosage (10)].
5.10 Use in Patients with Renal Dysfunction
Approximately 50% of propafenone metabolites are excreted in the urine following administration of propafenone hydrochloride immediate-release tablets. No trials have been performed to assess the percentage of metabolites eliminated in the urine following the administration of propafenone hydrochloride extended-release capsules.
In patients with impaired renal function, monitor for signs of overdosage [see Overdosage (10)].
5.11 Use in Patients with Myasthenia Gravis
Exacerbation of myasthenia gravis has been reported during propafenone therapy.
5.12 Elevated ANA Titers
Positive ANA titers have been reported in patients receiving propafenone. They have been reversible upon cessation of treatment and may disappear even in the face of continued propafenone therapy. These laboratory findings were usually not associated with clinical symptoms, but there is one published case of drug-induced lupus erythematosis (positive rechallenge); it resolved completely upon discontinuation of therapy. Carefully evaluate patients who develop an abnormal ANA test and, if persistent or worsening elevation of ANA titers is detected, consider discontinuing therapy.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to propafenone hydrochloride extended-release capsules 225 mg twice daily in 126 patients, to propafenone hydrochloride extended-release capsules 325 mg twice daily in 135 patients, to propafenone hydrochloride extended-release capsules 425 mg twice daily in 136 patients, and to placebo in 126 patients for up to 39 weeks (mean: 20 weeks) in a placebo- controlled trial (RAFT) conducted in the U.S. The most commonly reported adverse events with propafenone (greater than 5% and greater than placebo) excluding those not reasonably associated with the use of the drug or because they were associated with the condition being treated, were dizziness, palpitations, chest pain, dyspnea, taste disturbance, nausea, fatigue, anxiety, constipation, upper respiratory tract infection, edema, and influenza. The frequency of discontinuation due to adverse events was 17%, and the rate was highest during the first 14 days of treatment.
Cardiac-related adverse events occurring in greater than or equal to 2% of the patients in any of the RAFT propafenone SR treatment groups and more common with propafenone than with placebo, excluding those that are common in the population and those not plausibly related to drug therapy, included the following: angina pectoris, atrial flutter, AV block first-degree, bradycardia, congestive cardiac failure, cardiac murmur, edema, dyspnea, rales, wheezing, and cardioactive drug level above therapeutic.
Propafenone prolongs the PR and QRS intervals in patients with atrial and ventricular arrhythmias. Prolongation of the QRS interval makes it difficult to interpret the effect of propafenone on the QT interval [see Clinical Pharmacology (12.2)].
Non-cardiac related adverse events occurring in greater than or equal to 2% of the patients in any of the RAFT propafenone SR treatment groups and more common with propafenone than with placebo, excluding those that are common in the population and those not plausibly related to drug therapy, included the following: blurred vision, constipation, diarrhea, dry mouth, flatulence, nausea, vomiting, fatigue, weakness, upper respiratory tract infection, blood alkaline phosphatase increased, hematuria, muscle weakness, dizziness (excluding vertigo), headache, taste disturbance, tremor, somnolence, anxiety, depression, ecchymosis.
No clinically important differences in incidence of adverse reactions were noted by age or gender. Too few non-Caucasian patients were enrolled to assess adverse events according to race.
Adverse events occurring in 2% or more of the patients in any of the ERAFT [see Clinical Studies (14)] propafenone SR treatment groups and not listed above include the following: bundle branch block left, bundle branch block right, conduction disorders, sinus bradycardia, and hypotension.
Other adverse events reported with propafenone clinical trials not already listed elsewhere in the prescribing information include the following adverse events by body system and preferred term.
Blood and Lymphatic System
Anemia, lymphadenopathy, spleen disorder, thrombocytopenia.
Cardiac
Unstable angina, atrial hypertrophy, cardiac arrest, coronary artery disease, extrasystoles, myocardial infarction, nodal arrhythmia, palpitations, pericarditis, sinoatrial block, sinus arrest, sinus arrhythmia, supraventricular extrasystoles, ventricular extrasystoles, ventricular hypertrophy.
Ear and Labyrinth
Hearing impaired, tinnitus, vertigo.
Eye
Eye hemorrhage, eye inflammation, eyelid ptosis, miosis, retinal disorder, visual acuity reduced.
Gastrointestinal
Abdominal distension, abdominal pain, duodenitis, dyspepsia, dysphagia, eructation, gastritis, gastroesophageal reflux disease, gingival bleeding, glossitis, glossodynia, gum pain, halitosis, intestinal obstruction, melena, mouth ulceration, pancreatitis, peptic ulcer, rectal bleeding, sore throat.
General Disorders and Administration Site Conditions
Chest pain, feeling hot, hemorrhage, malaise, pain, pyrexia.
Hepatobiliary
Hepatomegaly.
Investigations
Abnormal heart sounds, abnormal pulse, carotid bruit, decreased blood chloride, decreased blood pressure, decreased blood sodium, decreased hemoglobin, decreased neutrophil count, decreased platelet count, decreased prothrombin level, decreased red blood cell count, decreased weight, glycosuria present, increased alanine aminotransferase, increased aspartate aminotransferase, increased blood bilirubin, increased blood cholesterol, increased blood creatinine, increased blood glucose, increased blood lactate dehydrogenase, increased blood pressure, increased blood prolactin, increased blood triglycerides, increased blood urea, increased blood uric acid, increased eosinophil count, increased gamma-glutamyltransferase, increased monocyte count, increased prostatic specific antigen, increased prothrombin level, increased weight, increased white blood cell count, ketonuria present, proteinuria present.
Metabolism and Nutrition
Anorexia, dehydration, diabetes mellitus, gout, hypercholesterolemia, hyperglycemia, hyperlipidemia, hypokalemia.
Musculoskeletal, Connective Tissue, and Bone
Arthritis, bursitis, collagen-vascular disease, costochondritis, joint disorder, muscle cramps, muscle spasms, myalgia, neck pain, pain in jaw, sciatica, tendonitis.
Nervous System
Amnesia, ataxia, balance impaired, brain damage, cerebrovascular accident, dementia, gait abnormal, hypertonia, hypothesia, insomnia, paralysis, paresthesia, peripheral neuropathy, speech disorder, syncope, tongue hypoesthesia.
Psychiatric
Decreased libido, emotional disturbance, mental disorder, neurosis, nightmare, sleep disorder.
Renal and Urinary
Dysuria, nocturia, oliguria, pyuria, renal failure, urinary casts, urinary frequency, urinary incontinence, urinary retention, urine abnormal.
Reproductive System and Breast
Breast pain, impotence, prostatism.
Respiratory, Thoracic, and Mediastinal
Atelectasis, breath sounds decreased, chronic obstructive airways disease, cough, epistaxis, hemoptysis, lung disorder, pleural effusion, pulmonary congestion, rales, respiratory failure, rhinitis, throat tightness.
Skin and Subcutaneous Tissue
Alopecia, dermatitis, dry skin, erythema, nail abnormality, petechiae, pruritus, sweating increased, urticaria.
Vascular
Arterial embolism limb, deep limb venous thrombosis, flushing, hematoma, hypertension, hypertensive crisis, hypotension, labile blood pressure, pallor, peripheral coldness, peripheral vascular disease, thrombosis.
-
7 DRUG INTERACTIONS
7.1 CYP2D6 and CYP3A4 Inhibitors
Drugs that inhibit CYP2D6 (such as desipramine, paroxetine, ritonavir, sertraline) and CYP3A4 (such as ketoconazole, ritonavir, saquinavir, erythromycin, grapefruit juice) can be expected to cause increased plasma levels of propafenone. The combination of CYP3A4 inhibition and either CYP2D6 deficiency or CYP2D6 inhibition with administration of propafenone may increase the risk of adverse reactions, including proarrhythmia. Therefore, simultaneous use of propafenone hydrochloride extended-release capsules with both a CYP2D6 inhibitor and a CYP3A4 inhibitor should be avoided [see Warnings and Precautions (5.4), Dosage and Administration (2)].
Amiodarone
Concomitant administration of propafenone and amiodarone can affect conduction and repolarization and is not recommended.
Cimetidine
Concomitant administration of propafenone immediate-release tablets and cimetidine in 12 healthy subjects resulted in a 20% increase in steady-state plasma concentrations of propafenone.
Fluoxetine
Concomitant administration of propafenone and fluoxetine in extensive metabolizers increased the S-propafenone Cmax and AUC by 39% and 50%, respectively, and the R-propafenone Cmax and AUC by 71% and 50%, respectively.
Quinidine
Small doses of quinidine completely inhibit the CYP2D6 hydroxylation metabolic pathway, making all patients, in effect, slow metabolizers [see Clinical Pharmacology (12.3)]. Concomitant administration of quinidine (50 mg 3 times daily) with 150 mg immediate-release propafenone 3 times daily decreased the clearance of propafenone by 60% in extensive metabolizers, making them poor metabolizers. Steady-state plasma concentrations increased by more than 2-fold for propafenone and decreased 50% for 5-OH-propafenone. A 100 mg dose of quinidine increased steady-state concentrations of propafenone 3-fold. Avoid concomitant use of propafenone and quinidine.
Rifampin
Concomitant administration of rifampin and propafenone in extensive metabolizers decreased the plasma concentrations of propafenone by 67% with a corresponding decrease of 5-OH-propafenone by 65%. The concentrations of norpropafenone increased by 30%. In poor metabolizers, there was a 50% decrease in propafenone plasma concentrations and an increase in the AUC and Cmax of norpropafenone by 74% and 20%, respectively. Urinary excretion of propafenone and its metabolites decreased significantly. Similar results were noted in elderly patients: Both the AUC and Cmax of propafenone decreased by 84%, with a corresponding decrease in AUC and Cmax of 5-OH-propafenone by 69% and 57%, respectively.
7.2 Digoxin
Concomitant use of propafenone and digoxin increased steady-state serum digoxin exposure (AUC) in patients by 60% to 270% and decreased the clearance of digoxin by 31% to 67%. Monitor plasma digoxin levels of patients receiving propafenone and adjust digoxin dosage as needed.
7.3 Warfarin
The concomitant administration of propafenone and warfarin increased warfarin plasma concentrations at steady state by 39% in healthy volunteers and prolonged the prothrombin time (PT) in patients taking warfarin. Adjust the warfarin dose as needed by monitoring INR (international normalized ratio).
7.4 Orlistat
Orlistat may limit the fraction of propafenone available for absorption. In postmarketing reports, abrupt cessation of orlistat in patients stabilized on propafenone has resulted in severe adverse events including convulsions, atrioventricular block, and acute circulatory failure.
7.5 Beta-Antagonists
Concomitant use of propafenone and propranolol in healthy subjects increased propranolol plasma concentrations at steady state by 113%. In 4 patients, administration of metoprolol with propafenone increased the metoprolol plasma concentrations at steady state by 100% to 400%. The pharmacokinetics of propafenone was not affected by the coadministration of either propranolol or metoprolol. In clinical trials using propafenone immediate-release tablets, patients who were receiving beta-blockers concurrently did not experience an increased incidence of side effects.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
In the absence of studies in pregnant women, available data from published case reports and several decades of postmarketing experience with use of propafenone hydrochloride in pregnancy have not identified any drug-associated risks of miscarriage, birth defects, or adverse maternal or fetal outcomes. Untreated arrhythmias during pregnancy may pose a risk to the pregnant woman and fetus (see Clinical Considerations). Propafenone and its metabolite, 5-OH-propafenone, cross the placenta in humans. In animal studies, propafenone was not teratogenic. At maternally toxic doses (ranging from 2 to 6 times the maximum recommended human dose [MRHD]), there was evidence of adverse developmental outcomes when administered to pregnant rabbits and rats during organogenesis or when administered to pregnant rats during mid-gestation through weaning of their offspring (see Data).
The estimated background risks of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk: The incidence of VT is increased and may be more symptomatic during pregnancy. Ventricular arrhythmias most often occur in pregnant women with underlying cardiomyopathy, congenital heart disease, valvular heart disease, or mitral valve prolapse. Breakthrough arrhythmias may also occur during pregnancy, as therapeutic treatment levels may be difficult to maintain due to the increased volume of distribution and increased drug metabolism inherent in the pregnant state.
Fetal/Neonatal Adverse Reactions: Propafenone and its metabolite have been shown to cross the placenta. Adverse reactions such as fetal/neonatal arrhythmias have been associated with the use of other antiarrhythmic agents by pregnant women. Fetal/neonatal monitoring for signs and symptoms of arrhythmia is recommended during and after treatment of pregnant women with propafenone.
Labor or Delivery: Risk of arrhythmias may increase during labor and delivery. Patients treated with propafenone hydrochloride should be monitored continuously for arrhythmias during labor and delivery [see Warnings and Precautions (5.1)].
Data
Propafenone has been shown to cause embryo-fetal mortality in rabbits and rats when given orally during organogenesis at maternally toxic doses of 150 mg/kg/day (rabbit: maternal mortality, decreased body weight gain and food consumption at approximately 3 times the MRHD on a mg/m2 basis) and 600 mg/kg/day (rat: maternal decreased body weight gain and food consumption at approximately 6 times the MRHD on a mg/m2 basis). In addition, a maternally toxic dose of 600 mg/kg/day (approximately 6 times the MRHD on a mg/m2 basis) also caused decreased fetal weights in rats. Increased placental weights and delayed ossification occurred in rabbits at a dose of 30 mg/kg/day (less than the MRHD on a mg/m2 basis) in the absence of maternal toxicity. No adverse developmental outcomes in the absence of maternal toxicity were seen following oral doses of 15 mg/kg/day to rabbits or up to 270 mg/kg/day to rats administered during organogenesis (equivalent to 0.3 times or approximately 3 times the MRHD on a mg/m2 basis, respectively). In an oral study, female rats received propafenone up to 500 mg/kg/day from mid-gestation through weaning. At 90 mg/kg/day (equivalent to the MRHD on a mg/m2 basis), there were no adverse developmental outcomes in the absence of maternal toxicity. However, doses ≥180 mg/kg/day (2 or more times the MRHD on a mg/m2 basis) produced increases in maternal deaths and resulted in reductions in neonatal survival, body weight gain, and delayed development in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
Propafenone and its active metabolite, 5-OH-propafenone, are present in human milk, but the levels are likely to be low. There are no data on the effects of propafenone on the breastfed infant or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for propafenone and any potential adverse effects on the breastfed infant from propafenone or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Males: Based on human and animal studies, propafenone hydrochloride may transiently impair spermatogenesis in males. Evaluation of the effects on spermatogenesis was performed in 11 healthy males given oral propafenone 300 mg b.i.d. for 4 days, which was then increased to 300 mg t.i.d. for an additional 4 days. Study findings included a 28% reduction in semen sample volume on Treatment Day 8 and a 27% reduction in sperm count 64 days after treatment (both values remained within the laboratories normal reference range). These effects were not seen in follow-up visits up to 120 days after treatment. Reversible decreases in spermatogenesis have been demonstrated in monkeys, dogs, and rabbits after lethal or near-lethal intravenous doses of propafenone [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of propafenone in pediatric patients have not been established.
8.5 Geriatric Use
Of the total number of subjects in Phase 3 clinical trials of propafenone hydrochloride extended-release capsules 46% were 65 and older, while 16% were 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals at higher doses cannot be ruled out. The effect of age on the pharmacokinetics and pharmacodynamics of propafenone has not been studied.
-
10 OVERDOSAGE
The symptoms of overdosage may include hypotension, somnolence, bradycardia, intra-atrial and intraventricular conduction disturbances, and rarely, convulsions and high-grade ventricular arrhythmias. Defibrillation, as well as infusion of dopamine and isoproterenol, has been effective in controlling abnormal rhythm and blood pressure. Convulsions have been alleviated with intravenous diazepam. General supportive measures such as mechanical respiratory assistance and external cardiac massage may be necessary.
The hemodialysis of propafenone in patients with an overdose is expected to be of limited value in the removal of propafenone as a result of both its high protein binding (greater than 95%) and large volume of distribution.
-
11 DESCRIPTION
Propafenone hydrochloride is an antiarrhythmic drug supplied in extended-release capsules of 225 mg, 325 mg, and 425 mg for oral administration.
Chemically, propafenone hydrochloride is 2’-[2-hydroxy-3-(propylamino)-propoxy]-3- phenylpropiophenone hydrochloride, with a molecular weight of 377.92. The molecular formula is C21H27NO3HCl.
Propafenone hydrochloride has some structural similarities to beta-blocking agents. The structural formula of propafenone hydrochloride is given below:
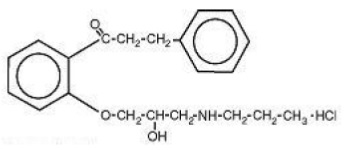
Propafenone hydrochloride, USP occurs as white or almost white powder or colorless crystals. It is soluble in methanol and hot water, slightly soluble in 96% ethanol and cold water. Propafenone hydrochloride extended-release capsules USP are filled with 2 mm white to off white round biconvex microtablets containing propafenone and the following inactive ingredients: gelatin, hypromellose, magnesium stearate, polyethylene glycol, red iron oxide, shellac, sodium lauryl sulphate and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Propafenone is a Class 1C antiarrhythmic drug with local anesthetic effects and a direct stabilizing action on myocardial membranes. The electrophysiological effect of propafenone manifests itself in a reduction of upstroke velocity (Phase 0) of the monophasic action potential. In Purkinje fibers, and, to a lesser extent, myocardial fibers, propafenone reduces the fast inward current carried by sodium ions. Diastolic excitability threshold is increased and effective refractory period prolonged. Propafenone reduces spontaneous automaticity and depresses triggered activity.
Studies in anesthetized dogs and isolated organ preparations show that propafenone has beta-sympatholytic activity at about 1/50 the potency of propranolol. Clinical studies employing isoproterenol challenge and exercise testing after single doses of propafenone indicate a beta-adrenergic blocking potency (per mg) about 1/40 that of propranolol in man. In clinical trials with the immediate-release formulation, resting heart rate decreases of about 8% were noted at the higher end of the therapeutic plasma concentration range. At very high concentrations in vitro, propafenone can inhibit the slow inward current carried by calcium, but this calcium antagonist effect probably does not contribute to antiarrhythmic efficacy. Moreover, propafenone inhibits a variety of cardiac potassium currents in in vitro studies (i.e., the transient outward, the delayed rectifier, and the inward rectifier current). Propafenone has local anesthetic activity approximately equal to procaine. Compared with propafenone, the main metabolite, 5- hydroxypropafenone, has similar sodium and calcium channel activity, but about 10 times less beta-blocking activity. (N-depropylpropafenone has weaker sodium channel activity but equivalent affinity for beta-receptors.)
12.2 Pharmacodynamics
Cardiac Electrophysiology
Electrophysiology trials in patients with ventricular tachycardia have shown that propafenone prolongs atrioventricular conduction while having little or no effect on sinus node function. Both atrioventricular nodal conduction time (AH interval) and His-Purkinje conduction time (HV interval) are prolonged. Propafenone has little or no effect on the atrial functional refractory period, but AV nodal functional and effective refractory periods are prolonged. In patients with Wolff-Parkinson-White syndrome, propafenone hydrochloride immediate-release tablets reduce conduction and increase the effective refractory period of the accessory pathway in both directions.
Electrocardiograms: Propafenone prolongs the PR and QRS intervals. Prolongation of the QRS interval makes it difficult to interpret the effect of propafenone on the QT interval.
Table 1. Mean Change ± SD in 12-Lead Electrocardiogram Results (RAFT) a Calculated using Bazett’s correction factor.
Propafenone Hydrochloride Extended-Release Capsules Twice-Daily Dosing
Placebo
225 mg
325 mg
425 mg
n = 126
n = 135
n = 136
n = 126
PR (ms)
9 ± 22
12 ± 23
21 ± 24
1 ± 16
QRS (ms)
4 ± 14
6 ± 15
6 ± 15
-2 ± 12
Heart rate
5 ± 24
7 ± 23
2 ± 22
8 ± 27
QTca (ms)
2 ± 30
5 ± 36
6 ± 37
5 ± 35
In RAFT [see Clinical Studies (14)], the distribution of the maximum changes in QTc compared with baseline over the trial in each patient was similar in the groups receiving propafenone hydrochloride extended-release capsules 225 mg twice daily, 325 mg twice daily, and 425 mg twice daily, and placebo. Similar results were seen in the ERAFT trial.
Table 2. Number of Patients According to the Range of Maximum QTc Change Compared with Baseline over the Trial in Each Dose Group (RAFT Trial).
Range Maximum QTc Change
Propafenone Hydrochloride Extended-Release Capsules
Placebo
N = 100
n (%)
225 mg Twice Daily N = 119
n (%)
325 mg Twice Daily N = 129
n (%)
425 mg Twice Daily N = 123
n (%)
>20%
1 (1)
6 (5)
3 (2)
5 (4)
10 to 20%
19 (16)
28 (22)
32 (26)
24 (20)
0 ≤10%
99 (83)
95 (74)
88 (72)
91 (76)
Hemodynamics
Trials in humans have shown that propafenone exerts a negative inotropic effect on the myocardium. Cardiac catheterization trials in patients with moderately impaired ventricular function (mean CI: 2.61 L/min/m2) utilizing intravenous propafenone infusions (loading dose of 2 mg/kg over 10 min + followed by 2 mg/min for 30 min) that gave mean plasma concentrations of 3.0 mcg/mL (a dose that produces plasma levels of propafenone greater than recommended oral dosing) showed significant increases in pulmonary capillary wedge pressure, systemic and pulmonary vascular resistances, and depression of cardiac output and cardiac index.
12.3 Pharmacokinetics
Absorption/Bioavailability
Maximal plasma levels of propafenone are reached between 3 to 8 hours following the administration of propafenone hydrochloride extended-release capsules. Propafenone is known to undergo extensive and saturable presystemic biotransformation which results in a dose-dependent and dosage-form-dependent absolute bioavailability; e.g., a 150 mg immediate-release tablet had an absolute bioavailability of 3.4%, while a 300 mg immediate-release tablet had an absolute bioavailability of 10.6%. Absorption from a 300 mg solution dose was rapid, with an absolute bioavailability of 21.4%. At still larger doses, above those recommended, bioavailability of propafenone from immediaterelease tablets increased still further.
Relative bioavailability assessments have been performed between propafenone hydrochloride extended-release capsules and propafenone hydrochloride immediate-release tablets. In extensive metabolizers, the bioavailability of propafenone from the SR formulation was less than that of the immediate-release formulation as the more gradual release of propafenone from the prolonged-release preparations resulted in an increase of overall first-pass metabolism (see Metabolism). As a result of the increased first-pass effect, higher daily doses of propafenone were required from the SR formulation relative to the immediate-release formulation to obtain similar exposure to propafenone. The relative bioavailability of propafenone from the 325 mg twice-daily regimens of propafenone hydrochloride extended-release capsules approximates that of propafenone hydrochloride immediate-release 150 mg 3-times-daily regimen. Mean exposure to 5-hydroxypropafenone was about 20% to 25% higher after SR capsule administration than after immediate-release tablet administration.
Food increased the exposure to propafenone 4-fold after single-dose administration of 425 mg of propafenone hydrochloride extended-release capsules. However, in the multiple-dose trial (425 mg dose twice daily), the difference between the fed and fasted state was not significant.
Distribution
Following intravenous administration of propafenone, plasma levels decline in a bi-phasic manner consistent with a 2-compartment pharmacokinetic model. The average distribution half-life corresponding to the first phase was about 5 minutes. The volume of the central compartment was about 88 liters (1.1 L/kg) and the total volume of distribution about 252 liters.
In serum, propafenone is greater than 95% bound to proteins within the concentration range of 0.5 to 2 mcg/mL.
Metabolism
There are 2 genetically determined patterns of propafenone metabolism. In over 90% of patients, the drug is rapidly and extensively metabolized with an elimination half-life from 2 to 10 hours. These patients metabolize propafenone into 2 active metabolites: 5-hydroxypropafenone, which is formed by CYP2D6, and N-depropylpropafenone (norpropafenone), which is formed by both CYP3A4 and CYP1A2.
In less than 10% of patients, metabolism of propafenone is slower because the 5-hydroxy metabolite is not formed or is minimally formed. In these patients, the estimated propafenone elimination half-life ranges from 10 to 32 hours. Decreased ability to form the 5-hydroxy metabolite of propafenone is associated with a diminished ability to metabolize debrisoquine and a variety of other drugs, such as encainide, metoprolol, and dextromethorphan, whose metabolism is mediated by the CYP2D6 isozyme. In these patients, the N-depropylpropafenone metabolite occurs in quantities comparable to the levels occurring in extensive metabolizers.
As a consequence of the observed differences in metabolism, administration of propafenone hydrochloride extended-release capsules to slow and extensive metabolizers results in significant differences in plasma concentrations of propafenone, with slow metabolizers achieving concentrations about twice those of the extensive metabolizers at daily doses of 850 mg/day. At low doses the differences are greater, with slow metabolizers attaining concentrations about 3 to 4 times higher than extensive metabolizers. In extensive metabolizers, saturation of the hydroxylation pathway (CYP2D6) results in greater-than-linear increases in plasma levels following administration of propafenone hydrochloride extended-release capsules. In slow metabolizers, propafenone pharmacokinetics is linear. Because the difference decreases at high doses and is mitigated by the lack of the active 5-hydroxymetabolite in the slow metabolizers, and because steady-state conditions are achieved after 4 to 5 days of dosing in all patients, the recommended dosing regimen of propafenone hydrochloride extended-release capsules is the same for all patients. The larger inter-subject variability in blood levels requires that the dose of the drug be titrated carefully in patients with close attention paid to clinical and ECG evidence of toxicity [see Dosage and Administration (2)].
The 5-hydroxypropafenone and norpropafenone metabolites have electrophysiologic properties similar to propafenone in vitro. In man after administration of propafenone hydrochloride extended-release capsules, the 5-hydroxypropafenone metabolite is usually present in concentrations less than 40% of propafenone. The norpropafenone metabolite is usually present in concentrations less than 10% of propafenone.
Inter-Subject Variability: With propafenone, there is a considerable degree of inter-subject variability in pharmacokinetics which is due in large part to the first-pass hepatic effect and nonlinear pharmacokinetics in extensive metabolizers. A higher degree of inter-subject variability in pharmacokinetic parameters of propafenone was observed following both single- and multiple-dose administration of propafenone hydrochloride extended-release capsules. Inter-subject variability appears to be substantially less in the poor-metabolizer group than in the extensive-metabolizer group, suggesting that a large portion of the variability is intrinsic to CYP2D6 polymorphism rather than to the formulation.
Stereochemistry: Propafenone hydrochloride is a racemic mixture. The R- and S-enantiomers of propafenone display stereoselective disposition characteristics. In vitro and in vivo studies have shown that the R-isomer of propafenone is cleared faster than the S-isomer via the 5-hydroxylation pathway (CYP2D6). This results in a higher ratio of S-propafenone to R-propafenone at steady state. Both enantiomers have equivalent potency to block sodium channels; however, the S-enantiomer is a more potent beta-antagonist than the R-enantiomer. Following administration of propafenone hydrochloride immediate-release tablets or propafenone hydrochloride extended-release capsules, the S/R ratio for the area under the plasma concentration-time curve was about 1.7. The S/R ratios of propafenone obtained after administration of 225 mg, 325 mg, and 425 mg propafenone hydrochloride extended-release capsules are independent of dose. In addition, no difference in the average values of the S/R ratios is evident between genotypes or over time.
Specific Populations
Patients with Hepatic Impairment: Decreased liver function increases the bioavailability of propafenone. Absolute bioavailability assessments have not been determined for the capsule formulation of propafenone hydrochloride extended-release capsules. Absolute bioavailability of propafenone hydrochloride immediate-release tablets is inversely related to indocyanine green clearance, reaching 60% to 70% at clearances of 7 mL/min and below. Protein binding decreases to about 88% in patients with severe hepatic dysfunction. The clearance of propafenone is reduced and the elimination half-life increased in patients with significant hepatic dysfunction [see Warnings and Precautions (5.9)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime maximally tolerated oral dose studies in mice (up to 360 mg/kg/day, approximately twice the MRHD on a mg/m2 basis) and rats (up to 270 mg/kg/day, approximately 3 times the MRHD on a mg/m2 basis) provided no evidence of a carcinogenic potential for propafenone. Propafenone was not mutagenic in the Ames (salmonella) test and the in vivo mouse dominant lethal test. Propafenone was not clastogenic in the human lymphocyte chromosome aberration assay in vitro, the rat and Chinese hamster micronucleus tests, and other in vivo tests for chromosomal aberrations in rat bone marrow and Chinese hamster bone marrow and spermatogonia.
Propafenone, administered intravenously, has been shown to decrease spermatogenesis at lethal doses in rabbits (≥3.5 mg/kg/day) or at near-lethal dose levels in monkeys and dogs (≤5 mg/kg/day); doses were less than the MRHD on a mg/m2 basis. These effects were reversible and did not impair fertility in rabbits at an intravenous dose of 3.5 mg/kg/day (a spermatogenesis-impairing dose). Effects on spermatogenesis were not found when propafenone was administered to rats either orally or intravenously up to 360 mg/kg/day or 6 mg/kg/day, respectively, or in dogs at oral doses up to 240 mg/kg/day (up to approximately 4 or 9 times the MRHD on a mg/m2 basis in rats and dogs, respectively). Treatment of male rabbits for 10 weeks prior to mating at an oral dose of 120 mg/kg/day (approximately 2 times the MRHD on a mg/m2 basis) did not result in evidence of impaired fertility. Nor was there evidence of impaired fertility when propafenone was administered orally to male and female rats at dose levels up to 270 mg/kg/day (approximately 3 times the MRHD on a mg/m2 basis) for 10 weeks (males) or 2 weeks (females) prior to mating through mating.
13.2 Animal Toxicology and/or Pharmacology
Renal changes have been observed in the rat following 6 months of oral administration of propafenone hydrochloride at doses of 180 and 360 mg/kg/day (about 2 and 4 times, respectively, the MRHD on a mg/m2 basis). Both inflammatory and non-inflammatory changes in the renal tubules, with accompanying interstitial nephritis, were observed. These changes were reversible, as they were not found in rats allowed to recover for 6 weeks. Fatty degenerative changes of the liver were found in rats following longer durations of administration of propafenone hydrochloride at a dose of 270 mg/kg/day (about 3 times the MRHD on a mg/m2 basis). There were no renal or hepatic changes at 90 mg/kg/day equivalent to the MRHD on a mg/m2 basis).
-
14 CLINICAL STUDIES
Propafenone hydrochloride extended-release capsules has been evaluated in patients with a history of electrocardiographically documented recurrent episodes of symptomatic AF in 2 randomized, double-blind, placebo-controlled trials.
RAFT
In one U.S. multicenter trial (RAFT), 3 doses of propafenone hydrochloride extended-release capsules (225 mg twice daily, 325 mg twice daily, and 425 mg twice daily) and placebo were compared in 523 patients with symptomatic, episodic AF. The patient population in this trial was 59% male with a mean age of 63 years, 91% white, and 6% black. The patients had a median history of AF of 13 months and documented symptomatic AF within 12 months of trial entry. Over 90% were NYHA Class I, and 21% had a prior electrical cardioversion. At baseline, 24% were treated with calcium channel blockers, 37% with beta-blockers, and 38% with digoxin. Symptomatic arrhythmias after randomization were documented by transtelephonic electrocardiogram and centrally read and adjudicated by a blinded adverse event committee. Propafenone hydrochloride extended-release capsules administered for up to 39 weeks was shown to prolong significantly the time to the first recurrence of symptomatic atrial arrhythmia, predominantly AF, from Day 1 of randomization (primary efficacy variable) compared with placebo, as shown in Table 3.
Table 3. Analysis of Tachycardia-Free Period (Days) from Day 1 of Randomization a Terminating events comprised 91% AF, 5% atrial flutter, and 4% PSVT.
b Not Applicable: Fewer than 50% of the patients had events. The median time is not calculable.Parameter
Dose of Propafenone Hydrochloride Extended-Release Capsules
225 mg Twice Daily
(N = 126)
n (%)
325 mg Twice Daily
(N = 135)
n (%)
425 mg Twice Daily
(N = 136)
n (%)
Placebo
(N = 126)
n (%)
Patients completing with terminating eventa
66 (52)
56 (41)
41 (30)
87 (69)
Comparison of tachycardia-free periods
Kaplan-Meier Media
112
291
NAb
41
Range
0 to 285
0 to 293
0 to 300
0 to 289
P-Value (Log-rank test)
0.014
<0.0001
<0.0001
--
Hazard Ratio compared with placebo
0.67
0.43
0.35
--
95% CI for Hazard Ratio
(0.49, 0.93)
(0.31, 0.61)
(0.24, 0.51)
--
There was a dose response for propafenone hydrochloride extended-release capsules for the tachycardia-free period as shown in the proportional hazard analysis and the Kaplan-Meier curves presented in Figure 1.
Figure 1. RAFT Kaplan-Meier Analysis for the Tachycardia-Free Period from Day 1 of Randomization
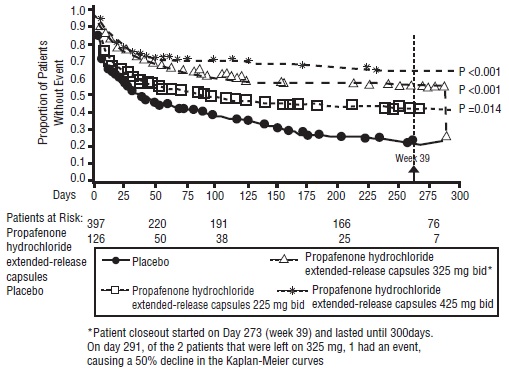
In additional analyses, propafenone hydrochloride extended-release capsules (225 mg twice daily, 325 mg twice daily, and 425 mg twice daily) was also shown to prolong time to the first recurrence of symptomatic AF from Day 5 (steady-state pharmacokinetics were attained). The antiarrhythmic effect of propafenone hydrochloride extended-release capsules was not influenced by age, gender, history of cardioversion, duration of AF, frequency of AF, or use of medication that lowers heart rate. Similarly, the antiarrhythmic effect of propafenone hydrochloride extended-release capsules was not influenced by the individual use of calcium channel blockers, beta-blockers, or digoxin. Too few non-white patients were enrolled to assess the influence of race on effects of propafenone hydrochloride extended-release capsules.
No difference in the average heart rate during the first recurrence of symptomatic arrhythmia between propafenone hydrochloride extended-release capsules and placebo was observed.
ERAFT
In a European multicenter trial (European Rythmonorm SR Atrial Fibrillation Trial [ERAFT]), 2 doses of propafenone hydrochloride extended-release capsules (325 mg twice daily and 425 mg twice daily) and placebo were compared in 293 patients with documented electrocardiographic evidence of symptomatic paroxysmal AF. The patient population in this trial was 61% male, 100% white with a mean age of 61 years. Patients had a median duration of AF of 3.3 years, and 61% were taking medications that lowered heart rate. At baseline, 15% of the patients were treated with calcium channel blockers (verapamil and diltiazem), 42% with beta-blockers, and 8% with digoxin. During a qualifying period of up to 28 days, patients had to have 1 ECG-documented incident of symptomatic AF. The double-blind treatment phase consisted of a 4-day loading period followed by a 91-day efficacy period. Symptomatic arrhythmias were documented by electrocardiogram monitoring.
In ERAFT, propafenone hydrochloride extended-release capsules was shown to prolong the time to the first recurrence of symptomatic atrial arrhythmia from Day 5 of randomization (primary efficacy analysis). The proportional hazard analysis revealed that both doses of propafenone hydrochloride extended-release capsules were superior to placebo. The antiarrhythmic effect of propafenone SR was not influenced by age, gender, duration of AF, frequency of AF, or use of medication that lowers heart rate. It was also not influenced by the individual use of calcium channel blockers, beta-blockers, or digoxin. Too few non-white patients were enrolled to assess the influence of race on the effects of propafenone hydrochloride extended-release capsules. There was a slight increase in the incidence of centrally diagnosed asymptomatic AF or atrial flutter in each of the 2 treatment groups receiving propafenone hydrochloride extended-release capsules compared with placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Propafenone Hydrochloride Extended-Release Capsules, USP 225 mg are white opaque cap/ white opaque body, size ‘1’ hard gelatin capsule shells, imprinted with “PRF 225” on cap in circular orientation with red ink.
Bottles of 60 NDC: 59651-276-60
Bottles of 90 NDC: 59651-276-90
Bottles of 500 NDC: 59651-276-05
Propafenone Hydrochloride Extended-Release Capsules, USP 325 mg are white opaque cap/ white opaque body, size ‘0’ hard gelatin capsule shells, imprinted with “PRF 325” on cap in circular orientation and single band around the circumference on the body with red ink.
Bottles of 60 NDC: 59651-277-60
Bottles of 90 NDC: 59651-277-90
Bottles of 500 NDC: 59651-277-05
Propafenone Hydrochloride Extended-Release Capsules, USP 425 mg are white opaque cap/ white opaque body, size ‘00’ hard gelatin capsule shells, imprinted with “PRF 425” on cap in circular orientation and triple band around the circumference on the body with red ink.
Bottles of 60 NDC: 59651-278-60
Bottles of 90 NDC: 59651-278-90
Bottles of 500 NDC: 59651-278-05
Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP using a child-resistant closure.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Instruct patients to notify their healthcare providers of any change in over-the-counter, prescription, and supplement use.
- Instruct patients to report symptoms that may be associated with altered electrolyte balance, such as excessive or prolonged diarrhea, sweating, vomiting, or loss of appetite or thirst.
- Instruct patients not to double the next dose if a dose is missed. The next dose should be taken at the usual time.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Revised: 10/2021 -
PATIENT INFORMATION
Propafenone Hydrochloride Extended-Release Capsules, USP
(proe'' pa fee none hye'' droe klor' ide)
What are propafenone hydrochloride extended-release capsules?
Propafenone hydrochloride extended-release capsules are a prescription medicine that is used:- in certain people who have a heart rhythm disorder called atrial fibrillation (AF)
- to increase the amount of time between having symptoms of AF
It is not known if propafenone hydrochloride extended-release capsules is safe and effective in children.
Who should not take propafenone hydrochloride extended-release capsules?
Do not take propafenone hydrochloride extended-release capsules if you have:- heart failure (weak heart)
- had a recent heart attack
- a heart condition called Brugada Syndrome
- a heart rate that is too slow, and you do not have a pacemaker
- very low blood pressure
- certain breathing problems that make you short of breath or wheeze
- certain abnormal body salt (electrolyte) levels in your blood
Talk to your doctor before taking propafenone hydrochloride extended-release capsules if you think you have any of the conditions listed above.
What should I tell my doctor before taking propafenone hydrochloride extended-release capsules?
Before you take propafenone hydrochloride extended-release capsules, tell your doctor if you:- have liver or kidney problems
- have breathing problems
- have symptoms including diarrhea, sweating, vomiting, or loss of appetite or thirst that are severe.
These symptoms may be a sign of abnormal electrolyte levels in your blood.
- have myasthenia gravis
- have lupus erythematosus
- have been told you have or had an abnormal blood test called Antinuclear Antibody Test or ANA Test
- are pregnant or plan to become pregnant
- are breastfeeding or plan to breastfeed. Propafenone hydrochloride extended-release capsules can pass into your milk. You and your doctor should discuss the best way to feed your baby during this time.
- have any other medical conditions
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Propafenone hydrochloride extended-release capsules and certain other medicines can affect each other and cause serious side effects. Propafenone hydrochloride extended-release capsules may affect the way other medicines work, and other medicines may affect how propafenone hydrochloride extended-release capsules works.
Especially tell your doctor if you take:
- amiodarone or other medicines for your abnormal heart beats
- an antidepressant medicine
- a medicine to treat anxiety
- ritonavir (for example, KALETRA, NORVIR) or saquinavir (for example, INVIRASE)
- an antibiotic medicine
- ketoconazole (for example, NIZORAL)
- digoxin (LANOXIN)
- warfarin sodium (for example, COUMADIN, JANTOVEN)
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take propafenone hydrochloride extended-release capsules?- Take propafenone hydrochloride extended-release capsules exactly as prescribed. Your doctor will tell you how many capsules to take and how often to take them.
- To help reduce the chance of certain side effects, your doctor may start you with a low dose of propafenone hydrochloride extended-release capsules, and then slowly increase the dose.
- Do not open or crush the capsule.
- You may take propafenone hydrochloride extended-release capsules with or without food.
- You should not drink grapefruit juice during treatment with propafenone hydrochloride extended-release capsules.
- If you miss a dose of propafenone hydrochloride extended-release capsules, take your next dose at the usual time. Do not take 2 doses at the same time.
- If you take too much propafenone hydrochloride, call your doctor or go to the nearest hospital emergency room right away.
- Call your doctor if your heart problems get worse.
What are possible side effects of propafenone hydrochloride extended-release capsules?
Propafenone hydrochloride extended-release capsules can cause serious side effects including:
- New or worsened abnormal heart beats, that can cause sudden death or be life-threatening. Your doctor may do an electrocardiogram (ECG or EKG) before and during treatment to check your heart for these problems.
-
New or worsened heart failure. Tell your doctor about any changes in your heart symptoms, including:
- any new or increased swelling in your arms or legs
- trouble breathing
- sudden weight gain
- Effects on pacemaker function. Propafenone hydrochloride extended-release capsules may affect how an implanted pacemaker or defibrillator works. Your doctor should check how your pacemaker or defibrillator is working during and after treatment with propafenone hydrochloride extended-release capsules. They may need to be re-programmed.
-
Very low white blood cell levels in your blood (agranulocytosis). Your bone marrow may not produce enough of a certain type of white blood cells called neutrophils. If this happens, you are more likely to get infections. Tell your doctor right away if you have any of these symptoms, especially during the first 3 months of treatment:
- fever
- sore throat
- chills
- Worsening of myasthenia gravis in people who already have this condition. Tell your doctor about any change in your symptoms.
- Propafenone hydrochloride extended-release capsules may cause lower sperm counts in men. This could affect the ability to father a child. Talk to your doctor if this is a concern for you.
Common side effects of propafenone hydrochloride extended-release capsules include:
- dizziness
- fast or irregular heart beats
- chest pain
- trouble breathing
- taste changes
- nausea
- tiredness
- feeling anxious
- constipation
- upper respiratory infection or flu
- swelling
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of propafenone hydrochloride extended-release capsules. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA- 1088.
How should I store propafenone hydrochloride extended-release capsules?
- Store propafenone hydrochloride extended-release capsules at room temperature between 20° to 25°C (68° to 77°F).
- Keep the bottle tightly closed.
Keep propafenone hydrochloride extended-release capsules and all medicines out of the reach of children.
General information about propafenone hydrochloride extended-release capsules
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use propafenone hydrochloride extended-release capsules for a condition for which it was not prescribed by your doctor. Do not give propafenone hydrochloride extended-release capsules to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about propafenone hydrochloride extended-release capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about propafenone hydrochloride extended-release capsules that is written for healthcare professionals. For more information about propafenone hydrochloride extended-release capsules, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
What are the ingredients in propafenone hydrochloride extended-release capsules?
Active Ingredient: Propafenone Hydrochloride
Inactive Ingredients: Gelatin, hypromellose, magnesium stearate, polyethylene glycol, red iron oxide, shellac, sodium lauryl sulphate and titanium dioxide.
All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Revised: 10/2021 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 225 mg (60 Capsules Bottle)
NDC: 59651-276-60
Propafenone Hydrochloride
Extended-Release
Capsules, USP
225 mg
Rx only 60 Capsules
AUROBINDO
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 325 mg (60 Capsules Bottle)
NDC: 59651-277-60
Propafenone Hydrochloride
Extended-Release
Capsules, USP
325 mg
Rx only 60 Capsules
AUROBINDO
-
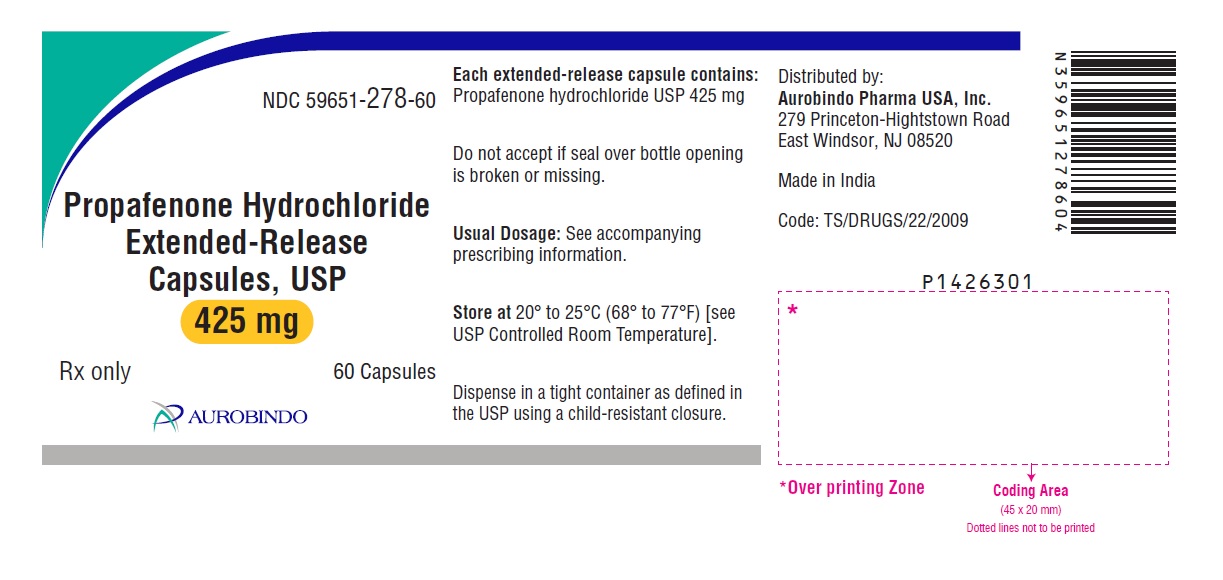
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 425 mg (60 Capsules Bottle)
NDC: 59651-278-60
Propafenone Hydrochloride
Extended-Release
Capsules, USP
425 mg
Rx only 60 Capsules
AUROBINDO
-
INGREDIENTS AND APPEARANCE
PROPAFENONE HYDROCHLORIDE
propafenone hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59651-276 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPAFENONE HYDROCHLORIDE (UNII: 33XCH0HOCD) (PROPAFENONE - UNII:68IQX3T69U) PROPAFENONE HYDROCHLORIDE 225 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (Opaque) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code PRF225 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59651-276-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 2 NDC: 59651-276-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 3 NDC: 59651-276-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213096 02/21/2023 PROPAFENONE HYDROCHLORIDE
propafenone hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59651-277 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPAFENONE HYDROCHLORIDE (UNII: 33XCH0HOCD) (PROPAFENONE - UNII:68IQX3T69U) PROPAFENONE HYDROCHLORIDE 325 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (Opaque) , RED (Single band) Score no score Shape CAPSULE Size 21mm Flavor Imprint Code PRF325 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59651-277-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 2 NDC: 59651-277-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 3 NDC: 59651-277-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213096 02/21/2023 PROPAFENONE HYDROCHLORIDE
propafenone hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59651-278 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPAFENONE HYDROCHLORIDE (UNII: 33XCH0HOCD) (PROPAFENONE - UNII:68IQX3T69U) PROPAFENONE HYDROCHLORIDE 425 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FERRIC OXIDE RED (UNII: 1K09F3G675) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (Opaque) , RED (Three bands) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PRF425 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59651-278-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 2 NDC: 59651-278-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 3 NDC: 59651-278-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213096 02/21/2023 Labeler - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(59651-276, 59651-277, 59651-278) , MANUFACTURE(59651-276, 59651-277, 59651-278)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.