MUCINEX- guaifenesin tablet, extended release
Mucinex by
Drug Labeling and Warnings
Mucinex by is a Otc medication manufactured, distributed, or labeled by Aphena Pharma Solutions - Tennessee, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each extended-release bi-layer tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count 600 mg 60 67544-149-53 120 67544-149-70 180 67544-149-80 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
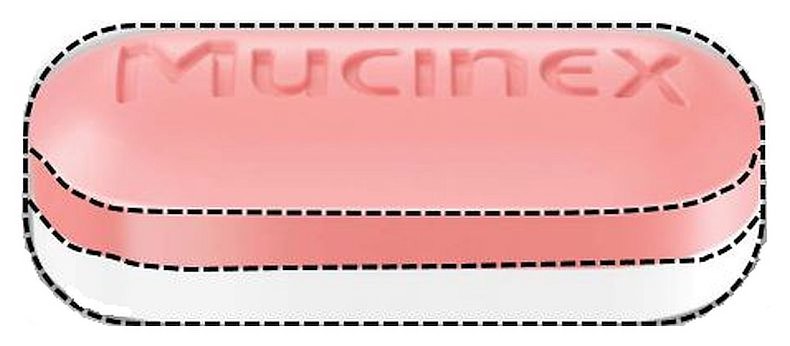
20171218DKJ - PRINCIPAL DISPLAY PANEL - 600mg
-
INGREDIENTS AND APPEARANCE
MUCINEX
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67544-149(NDC:63824-008) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE (blue and white) Score no score Shape OVAL Size 16mm Flavor Imprint Code Mucinex;600 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67544-149-53 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/09/2012 2 NDC: 67544-149-70 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2012 3 NDC: 67544-149-80 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/12/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021282 07/03/2012 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 REPACK(67544-149)
Trademark Results [Mucinex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MUCINEX 98499792 not registered Live/Pending |
RB Health (US) LLC 2024-04-15 |
 MUCINEX 90229382 not registered Live/Pending |
RB Health (US) LLC 2020-10-01 |
 MUCINEX 88040437 not registered Live/Pending |
Reckitt Benckiser LLC 2018-07-17 |
 MUCINEX 86191608 4613098 Live/Registered |
RB HEALTH (US) LLC 2014-02-12 |
 MUCINEX 85496330 4168070 Live/Registered |
RB HEALTH (US) LLC 2011-12-15 |
 MUCINEX 85489681 4168052 Live/Registered |
RB HEALTH (US) LLC 2011-12-07 |
 MUCINEX 77770135 3722363 Live/Registered |
Reckitt Benckiser Inc. 2009-06-29 |
 MUCINEX 76297961 2670161 Live/Registered |
RB HEALTH (US) LLC 2001-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
