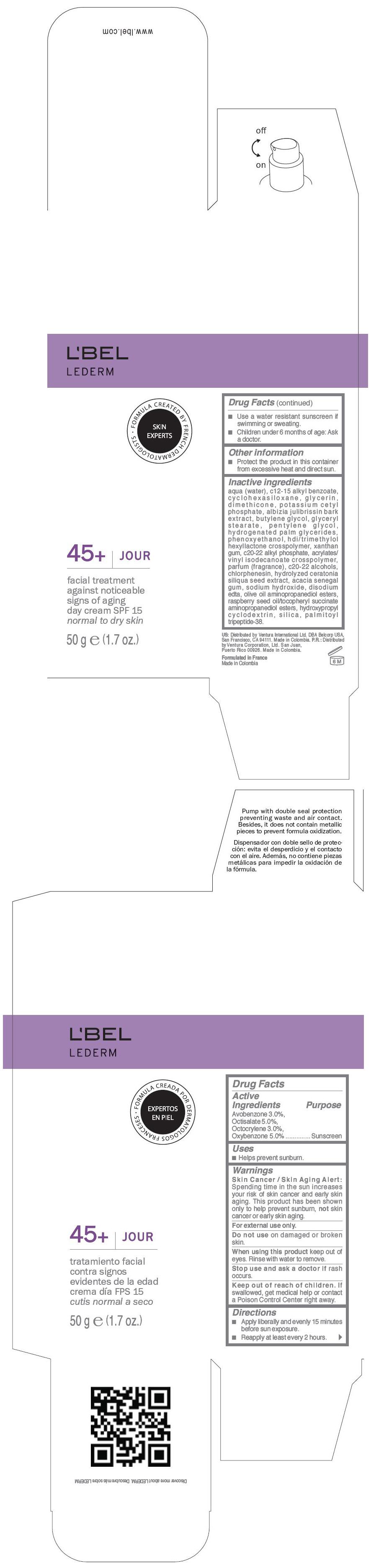
LBEL LEDERM 45PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST NOTICEABLE SIGNS OF AGING DAY - NORMAL TO DRY SKIN- avobenzone, octisalate, octocrylene, and oxybenzone cream
LBEL LEDERM 45plus Jour by
Drug Labeling and Warnings
LBEL LEDERM 45plus Jour by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD., Bel Star S.A. (Colombia), Yobel Supply Chain Management S.A. (Peru). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
AQUA (WATER), C12-15 ALKYL BENZOATE, CYCLOHEXASILOXANE, GLYCERIN, DIMETHICONE, POTASSIUM CETYL PHOSPHATE, ALBIZIA JULIBRISSIN BARK EXTRACT, BUTYLENE GLYCOL, GLYCERYL STEARATE, PENTYLENE GLYCOL, HYDROGENATED PALM GLYCERIDES, PHENOXYETHANOL, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, XANTHAN GUM, C20-22 ALKYL PHOSPHATE, ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER, PARFUM (FRAGRANCE), C20-22 ALCOHOLS, CHLORPHENESIN, HYDROLYZED CERATONIA SILIQUA SEED EXTRACT, ACACIA SENEGAL GUM, SODIUM HYDROXIDE, DISODIUM EDTA, OLIVE OIL AMINOPROPANEDIOL ESTERS, RASPBERRY SEED OIL/TOCOPHERYL SUCCINATE AMINOPROPANEDIOL ESTERS, HYDROXYPROPYL CYCLODEXTRIN, SILICA, PALMITOYL TRIPEPTIDE-38
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 g Bottle Carton
-
INGREDIENTS AND APPEARANCE
LBEL LEDERM 45PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST NOTICEABLE SIGNS OF AGING DAY - NORMAL TO DRY SKIN
avobenzone, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-447 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.03 g in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.05 g in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.03 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.05 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) ALBIZIA JULIBRISSIN BARK (UNII: 0J9G6W44DV) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) C20-22 ALKYL PHOSPHATE (UNII: L4VKP0Y7RP) C20-22 ALCOHOLS (UNII: O4M0347C6A) CHLORPHENESIN (UNII: I670DAL4SZ) ACACIA (UNII: 5C5403N26O) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE DISODIUM (UNII: 7FLD91C86K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-447-01 1 g in 1 PACKET 2 NDC: 13537-447-04 1 in 1 BOX 2 NDC: 13537-447-03 5 g in 1 BOTTLE, PLASTIC 3 NDC: 13537-447-06 1 in 1 BOX 3 NDC: 13537-447-05 50 g in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/01/2013 Labeler - Ventura Corporation LTD. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-447) Establishment Name Address ID/FEI Business Operations Yobel Supply Chain Management S.A. (Peru) 934116930 MANUFACTURE(13537-447)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.