These highlights do not include all the information needed to use LABETALOL HYDROCHLORIDE INJECTION, safely and effectively. See full prescribing information for LABETALOL HYDROCHLORIDE. LABETALOL HYDROCHLORIDE IN SODIUM CHLORIDE injection , for intravenous use. LABETALOL HYDROCHLORIDE IN DEXTROSE injection , for intravenous use. LABETALOL HYDROCHLORIDE injection, for intravenous use. Initial U.S. Approval: 1984
Labetalol Hydrochloride by
Drug Labeling and Warnings
Labetalol Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Hikma Pharmaceuticals USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LABETALOL HYDROCHLORIDE- labetalol hydrochloride injection
Hikma Pharmaceuticals USA Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LABETALOL HYDROCHLORIDE INJECTION, safely and effectively. See full prescribing information for LABETALOL HYDROCHLORIDE.
LABETALOL HYDROCHLORIDE IN SODIUM CHLORIDE injection, for intravenous use. LABETALOL HYDROCHLORIDE IN DEXTROSE injection, for intravenous use. LABETALOL HYDROCHLORIDE injection, for intravenous use. Initial U.S. Approval: 1984 RECENT MAJOR CHANGES5.6 Hypoglycemia 04/2023 INDICATIONS AND USAGELabetalol Hydrochloride (HCl) is a beta adrenergic blocker. Labetalol HCl injection is indicated in severe hypertension to lower blood pressure (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse events: To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. For product Inquiry call 1-877-845-0689. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 4/2024 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Labetalol HCl Injection is indicated in severe hypertension, to lower blood pressure.
2 DOSAGE AND ADMINISTRATION
2.1 General Information
Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection:
Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection are ready-to-use solutions and do not require further dilution. Check for leaks by squeezing the bag firmly. If leaks are found, discard solution, as sterility may be impaired. Do not add any additional medications to the bag.
Once infusion has started, discard any remaining at 24 hours.
2.2 Recommended Dosage
Choose intravenous administration by slow continuous infusion or repeated injection. The usual intravenous dose is in the range of 50 to 200 mg, but the safety of doses above 300 mg has not been established. Once supine diastolic blood pressure has begun to rise, transition to oral labetalol HCl.
Slow Continuous Infusion:Initiate at 2 mg/minute. Monitor blood pressure and adjust the dosage and duration of infusion accordingly.
Repeated Intravenous Injection:Administer 0.25 mg/kg up to 20 mg over 2 minutes. Administer 20 to 80 mg over 2 minutes at 10-minute intervals until a desired supine blood pressure is achieved. The maximum effect usually occurs within 5 minutes of each injection.
2.3 Instructions for Use of Labetalol HCl Injection Prefilled Syringe
CAUTION: Glass syringes may malfunction, break or clog when connected to some Needleless Luer Access Devices (NLADs) and needles. The external collar must remain attached to the syringe (See Figure 1). Spontaneous disconnection of this glass syringe from needles and NLADs with leakage of drug product may occur. Assure that the needle or NLAD is securely attached before beginning the injection. Inspect the glass syringe-needle or glass syringe –NLAD connection before and during drug administration.
Figure 1
Figure 1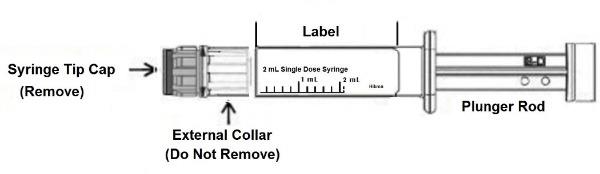
1. Push plunger rod slightly to break the stopper loose while tip cap is still on.
2. Remove tip cap by twisting it off. (See Figure 2)
Figure 2
Figure 2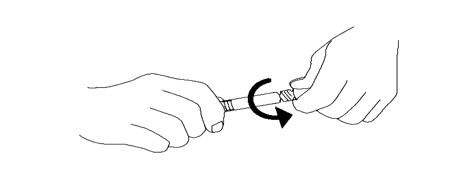
3. Connect the syringe to an appropriate injection connection.
4. Depress plunger rod to deliver the required dose.
3 DOSAGE FORMS AND STRENGTHS
Labetalol HCl in Sodium Chloride Injection is available as 1 mg/mL in 100-, 200-, or 300-mL bags.
Labetalol HCl in Dextrose Injection is available as 1 mg/mL in 200-mL bags.
Labetalol HCl Injection, USP, in a prefilled syringe is available as 10 mg in 2 mL
4 CONTRAINDICATIONS
Labetalol Hydrochloride Injection iscontraindicated in patients with:
- Bronchial asthma or obstructive airway disease.
- Severe sinus bradycardia:
- Heart block greater than first degree.
- Cardiogenic shock.
- IV administration of non-dihydropyridine calcium-channel antagonists (e.g., verapamil)
- Hypersensitivity reactions, including anaphylaxis, to labetalol
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Symptomatic postural hypotension (incidence, 58%) is likely to occur if patients are tilted or allowed to assume the upright position within 3 hours of receiving labetalol HCl injection. Before permitting any ambulation, establish patient’s ability to tolerate an upright position and observe the patient at the time of first ambulation.
5.2 Bradycardia
Bradycardia, including sinus pause, heart block, severe bradycardia, and cardiac arrest have occurred with the use of beta blockers. Monitor heart rate and rhythm in patients receiving labetalol hydrochloride injection.
5.3 Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure. Beta-blockade carries a potential hazard of further depressing myocardial contractility and precipitating more severe failure. Avoid labetalol HCl injection in patients with overt congestive heart failure. If patients develop signs or symptoms of heart failure during administration, discontinue labetalol and treat appropriately.
5.4 Ischemic Heart Disease
Abrupt cessation of therapy with beta blocking agents in patients with coronary artery disease, can cause exacerbations of angina pectoris and, in some cases, myocardial infarction has been reported. Therefore, even in the absence of overt angina pectoris, after the discontinuation of labetalol HCl injection observe patients for development or worsening of angina. If patient experiences angina or angina markedly worsens or if acute coronary insufficiency develops, promptly reinstitute labetalol HCl injection and manage as unstable angina.
5.5 Reactive Airway Disease and Nonallergic Bronchospasm
Patients with reactive airways disease should, in general, not receive beta blockers. Labetalol HCl at the usual intravenous therapeutic doses has not been studied in patients with nonallergic bronchospastic disease. In the event of bronchospasm, stop the infusion immediately, and treat as appropriate.
5.6 Hypoglycemia
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus or children and patients who are fasting (i.e., surgery, not eating regularly, or are vomiting). If severe hypoglycemia occurs, patients should be instructed to see medical treatment.
5.7 Use in Patients with Pheochromocytoma
Intravenous labetalol has been shown to lower blood pressure and relieve symptoms in patients with pheochromocytoma; higher than usual doses may be required. However, paradoxical hypertensive responses have been reported in a few patients with this tumor; therefore, monitor blood pressure when administering intravenous labetalol HCl to patients with pheochromocytoma.
5.8 Hepatic Injury
Severe hepatocellular injury occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis and death have been reported. If the patient develops signs or symptoms of liver injury, institute appropriate treatment and investigate the probable cause. Do not restart labetalol in patients without another explanation for the observed liver injury.
5.9 Use in Patients at Risk of Severe Acute Hypersensitivity Reactions
Patients at risk of anaphylactic reactions may be more reactive to allergen exposure (accidental, diagnostic, or therapeutic). Patients using beta-blockers may be unresponsive to the usual doses of epinephrine used to treat anaphylactic or anaphylactoid reactions. Avoid labetalol HCl injection in patients at high risk of anaphylactic reactions.
5.10 Intraoperative Floppy Iris Syndrome (IFIS)
IFIS has been observed during cataract surgery in some patients treated with alpha-1 blockers (labetalol is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combination of flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. Inform the patient’s ophthalmologist to be prepared for possible modifications to the surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypotension [see Warnings and Precautions (5.1)]
- Bradycardia [see Warnings and Precautions (5.2)]
- Depression of myocardial contractility in patients with overt congestive heart failure [see Warnings and Precautions (5.3)]
- Aggravation of angina [see Warnings and Precautions (5.4)]
- Significant decline in cardiac output following coronary bypass [see Warnings and Precautions (5.3)]
- Bronchospasm in patients with reactive airway disease [see Warnings and Precautions (5.5)]
- Paradoxical hypertensive responses in patients with pheochromocytoma [see Warnings and Precautions (5.7)]
- Hepatic injury [see Warnings and Precautions (5.8)]
- Acute hypersensitivity reaction [see Warnings and Precautions (5.9)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Most adverse effects have been mild and transient and, in controlled trials involving 92 patients, did not require labetalol withdrawal. Symptomatic postural hypotension (incidence, 58%) is likely to occur if patients are tilted or allowed to assume the upright position within 3 hours of receiving labetalol HCl. Moderate hypotension occurred in 1 of 100 patients while supine. Increased sweating was noted in 4 of 100 patients, and flushing occurred in 1 of 100 patients.
The following also were reported with labetalol HCl with the incidence as noted:
Central and Peripheral Nervous Systems
Dizziness in 9%
Paresthesia, most frequently described as tingling of the scalp/skin in 7%
Gastrointestinal System
Nausea in 13%
Vomiting in 4%
Metabolic Disorders
Transient increases in blood urea nitrogen and serum creatinine levels occurred in 8%; these were associated with drops in blood pressure, generally in patients with prior renal insufficiency.
Respiratory System
Bronchospasm
In addition, a number of other less common adverse events have been reported:
Cardiovascular:
Hypotension, and rarely, syncope, bradycardia, heart block.
Liver and Biliary System
Hepatic necrosis, hepatitis, cholestatic jaundice, elevated liver function tests.
Hypersensitivity
Rare reports of hypersensitivity (e.g., rash, urticaria, pruritus, angioedema, dyspnea) and anaphylactoid reactions.
The oculomucocutaneous syndrome associated with the beta blocker practolol has not been reported with labetalol HCl during investigational use and extensive foreign marketing experience.
Clinical Laboratory Tests
Among patients dosed with labetalol tablets, there have been reversible increases of serum transaminases in 4% of patients tested and, more rarely, reversible increases in blood urea.
7 DRUG INTERACTIONS
7.1 Bronchodilators
Labetalol HCl antagonizes the bronchodilatory effect of beta-receptor agonist drugs; therefore, labetalol HCl is contraindicated in patients with bronchial asthma [see Contraindications (4)].
7.2 Anesthesia
Synergism has been shown between halothane anesthesia and intravenously administered labetalol. During controlled hypotensive anesthesia using labetalol in association with halothane, high concentrations (3% or above) of halothane should not be used because the degree of hypotension will be increased and because of the possibility of a large reduction in cardiac output and an increase in central venous pressure.
7.3 Nitroglycerin
Coadministration of labetalol HCl and nitroglycerin will have an additive effect in lowering blood pressure. Additionally, labetalol HCl blunts the reflex tachycardia produced by nitroglycerin. If labetalol is used in patients with angina pectoris on nitroglycerin, monitor patients’ blood pressure and adjust labetalol HCl injection dose as needed. In these patients, avoid initiating labetalol HCl tablets.
7.4 Calcium Channel Blockers
Coadministration of labetalol HCl with non-dihydropyrindine calcium-channel antagonists (e.g., verapamil) is contraindicated [see Contraindications (4)]. Avoid the use of labetalol in patients receiving calcium-channel antagonists.
7.5 Drug/Laboratory Test Interactions
The presence of labetalol metabolites in the urine may result in falsely elevated levels of urinary catecholamines, metanephrine, normetanephrine, and vanillylmandelic acid (VMA) when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with labetalol, a specific method, such as a high-performance liquid chromatographic assay with solid phase extraction should be employed in determining levels of catecholamines.
Labetalol has also been reported to produce a false-positive test for amphetamine when screening urine for the presence of drugs using the commercially available assay methods. When patients being treated with labetalol have a positive urine test for amphetamine using these techniques, confirm using more specific methods, such as a gas chromatographic-mass spectrometer technique.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The extensive experience with use of labetalol in pregnant women, based on published interventional and observational studies, has not identified a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Untreated hypertension during pregnancy can lead to serious adverse outcomes for the mother and the fetus (see Clinical Considerations). In animal reproduction studies, oral administration of labetalol to pregnant rats and rabbits during organogenesis at doses up to approximately six and four times the maximum recommended human dose (MRHD), respectively, resulted in no fetal malformations; however, increased fetal resorptions were seen in both species at doses approximating the MRHD (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Labetalol crosses the placenta. Neonates born to mothers who are receiving labetalol during pregnancy, may be at risk for hypotension, bradycardia, hypoglycemia, and respiratory depression. Neonates should be monitored for symptoms of hypotension, bradycardia, hypoglycemia and respiratory depression and manage accordingly.
Data
Human Data
Data from published interventional and observational studies did not demonstrate an association between major congenital malformations and the use of labetalol in pregnancy, however, most studies reported the maternal use of intravenous labetalol occurring after 20 weeks gestation.The published literature has reported inconsistent findings of intrauterine growth retardation, preterm birth and perinatal mortality with maternal use of labetalol during pregnancy; however, these studies have methodological limitations hindering interpretation. These studies cannot definitively establish the absence of risk during pregnancy.
Animal Data
Teratogenic studies were performed with labetalol in rats and rabbits at oral doses up to approximately six and four times the maximum recommended human dose (MRHD), respectively. No reproducible evidence of fetal malformations was observed. Increased fetal resorptions were seen in both species at doses approximating the MRHD.
A teratology study performed with labetalol in rabbits at intravenous doses up to 1.7 times the MRHD revealed no evidence of drug-related harm to the fetus.
Oral administration of labetalol to rats during late gestation through weaning at doses of two to four times the MRHD caused a decrease in neonatal survival.
8.2 Lactation
Risk Summary
Available published data report the presence oflabetalol in human milk at low levels. There are no data on the effects on the breastfed infant and on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for labetalol and any potential adverse effects on the breastfed infant from labetalol or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Infertility
Based on the published literature, beta blockers, including labetalol, may cause erectile dysfunction and inhibit sperm motility.
8.5 Geriatric Use
Some pharmacokinetic studies indicate that the elimination of labetalol is reduced in elderly patients [see Clinical Pharmacology (12.3)]. Geriatric patients treated with labetalol could initiate therapy at the currently recommended dose of 2 mg/minute by continuous intravenous infusion; however, lower maintenance dosages are generally required for elderly patients than nonelderly patients. Monitor blood pressure and adjust the dosage and duration of infusion accordingly until the desired response is obtained [see Dosage and Administration (2)].
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
Overdosage with labetalol HCl causes excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary, to improve the blood supply to the brain. Treat symptoms of overdose with standard supportive care. If overdosage with labetalol HCl follows oral ingestion, gastric lavage or pharmacologically induced emesis (using syrup of ipecac) may be useful for removal of the drug shortly after ingestion.
Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol from the general circulation (<1%).
The oral LD50 value of labetalol HCl in the mouse is approximately 600 mg/kg and in the rat is greater than 2 g/kg. The intravenous LD50 in these species is 50 to 60 mg/kg.
11 DESCRIPTION
Labetalol HCl in Sodium Chloride Injection, Labetalol HCl in Dextrose Injection and Labetalol Hydrochloride Injection, USP contain labetalol HCl an adrenergic receptor blocking agent that has both selective alpha1-adrenergic and nonselective beta-adrenergic receptor blocking actions in a single substance. Labetalol hydrochloride (HCl) is a racemate chemically designated as 5-[1-Hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]-salicylamide monohydrochloride and it has the following structural formula:
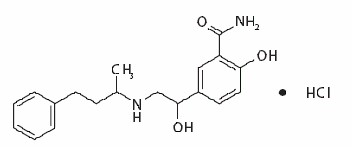
Labetalol HCl is a white or off-white crystalline powder, soluble in water. Labetalol HCl has the molecular formula C19H24N2O3HCl and a molecular weight of 364.87. It has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs.
Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection are two preservative-free, ready-to use formulations of labetalol.Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection are clear, colorless to light yellow, aqueous, sterile, isotonic solution for intravenous injection.
Each milliliter of Labetalol HCl in Sodium Chloride Injection contains 1 mg of labetalol HCl, 7.2 mg sodium chloride, 9 mg of anhydrous dextrose, 0.02 mg of edetate disodium; and citric acid monohydrate and sodium hydroxide, as necessary, to bring the solution into the pH range of 3.5 to 4.5.
Each milliliter of Labetalol HCl in Dextrose Injection contains 1 mg of labetalol HCl, 45 mg of anhydrous dextrose, 0.02 mg of edetate disodium; and citric acid monohydrate and sodium hydroxide, as necessary, to bring the solution into the pH range of 3.5 to 4.5.
Labetalol Hydrochloride Injection in a prefilled syringe is a preservative-free, ready-to use formulation of labetalol.Labetalol Hydrochloride Injection is a clear, colorless to light yellow, aqueous, sterile, isotonic solution for intravenous injection.
Each milliliter of the Labetalol Hydrochloride Injection, USP 2 mL prefilled syringe contains labetalol hydrochloride 5 mg; anhydrous dextrose 45 mg; edetate disodium 0.1 mg; citric acid monohydrate and sodium hydroxide as necessary to bring the pH into a range of 3.0 to 4.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Labetalol has both competitive alpha1-adrenergic blocking and competitive beta-adrenergic blocking activity. In man, the ratio of alpha- to beta-blockade has been estimated to be approximately 1:7 following intravenous administration. Beta2-agonist activity has been demonstrated in animals with minimal beta1-agonist activity detected.
12.2 Pharmacodynamics
In a clinical pharmacologic study in severe hypertensives, an initial 0.25 mg/kg injection of labetalol HCl administered to patients in the supine position decreased blood pressure by an average of 11/7 mmHg. Additional injections of 0.5 mg/kg at 15-minute intervals up to a total cumulative dose of 1.75 mg/kg of labetalol HCl caused further dose-related decreases in blood pressure. Some patients required cumulative doses of up to 3.25 mg/kg. The maximal effect of each dose level occurred within 5 minutes. Following discontinuation of intravenous treatment with labetalol HCl, the blood pressure rose gradually and progressively, approaching pretreatment baseline values within an average of 16 to 18 hours in the majority of patients.
Similar results were obtained in the treatment of patients with severe hypertension who required urgent blood pressure reduction with an initial dose of 20 mg (which corresponds to 0.25 mg/kg for an 80 kg patient) followed by additional doses of either 40 or 80 mg at 10 minute intervals to achieve the desired effect, or up to a cumulative dose of 300 mg.
Labetalol HCl administered as a continuous intravenous infusion, with a mean dose of 136 mg (27 to 300 mg) over a period of 2 to 3 hours (mean of 2 hours and 39 minutes), lowered the blood pressure by an average of 60/35 mmHg.
12.3 Pharmacokinetics
Distribution
Labetalol has been shown to cross the placental barrier in humans. Only negligible amounts of the drug crossed the blood-brain barrier in animal studies. Labetalol is approximately 50% protein bound. Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol from the systemic circulation (<1%).
Elimination
Following intravenous infusion of labetalol, the elimination half-life is about 5.5 hours and the total body clearance is approximately 33 mL/min/kg. Steady-state plasma levels of labetalol during repetitive dosing are reached following 22 to 28 hours of continuous infusion.
Metabolism
The metabolism of labetalol is mainly through conjugation to glucuronide metabolites.
Excretion
Approximately 55% to 60% of a dose appears in the urine as conjugates or unchanged labetalol within the first 24 hours of dosing. The metabolites are present in plasma and are excreted in the urine and, via the bile, into the feces.
Specific Populations
Patients with Renal or Hepatic Impairment
In patients with decreased hepatic or renal function, the elimination half-life of labetalol is not altered.
Geriatric Patients
Some pharmacokinetic studies indicate that the elimination of labetalol is reduced in elderly patients.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Long-term oral dosing studies with labetalol for 18 months in mice and for 2 years in rats showed no evidence of carcinogenesis. Studies with labetalol using dominant lethal assays in rats and mice and exposing microorganisms according to modified Ames tests showed no evidence of mutagenesis.
16 HOW SUPPLIED
16.1 How Supplied
Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection are preservative-free, clear, colorless to light yellow sterile solutions that are available in a single-dose single-port bag with an aluminum overwrap. The container closure is not made with natural rubber latex. It is available in the following presentations:
|
Product |
Strength |
Package |
NDC Number |
|
Labetalol HCl in Sodium Chloride Injection |
100 mg/100 mL (1 mg/mL) preservative-free |
1 single-dose bag |
0143-9363-01 |
|
Box of 10 bags |
0143-9363-10 |
||
|
Labetalol HCl in Sodium Chloride Injection |
200 mg/200 mL (1 mg/mL) preservative-free |
1 single-dose bag |
0143-9364-01 |
|
Box of 10 bags |
0143-9364-10 |
||
|
Labetalol HCl in Sodium Chloride Injection |
300 mg/300 mL (1 mg/mL) preservative-free |
1 single-dose bag |
0143-9365-01 |
|
Box of 10 bags |
0143-9365-10 |
||
|
Labetalol HCl in Dextrose Injection |
200 mg/200 mL (1 mg/mL) preservative-free |
1 single-dose bag |
0143-9366-01 |
|
Box of 10 bags |
0143-9366-10 |
Labetalol Hydrochloride Injection, USP is a preservative-free, clear, colorless to light yellow sterile solution that is available in a single-dose prefilled syringe. It is available in the following presentations:
|
Product |
Strength |
Package |
NDC Number |
|
Labetalol Hydrochloride Injection, USP |
10 mg/2 mL (5 mg/mL) preservative-free |
1 single-dose prefilled syringe |
0641-6252-01 |
|
Carton of 10 prefilled syringes |
0641-6252-10 |
16.2 Storage
Store at 20° to 25°C (68° to 77°F) with excursions permitted between 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature]. Do not freeze. Protect from light.
Labetalol HCl in Sodium Chloride Injection and Labetalol HCl in Dextrose Injection: Do not remove from overwrap until ready to use.
17 PATIENT COUNSELING INFORMATION
- Advise patients to remain supine and to proceed gradually in becoming ambulatory during and immediately following infusion (for up to 3 hours) of labetalol HCl injection.
- Inform patient to notify their healthcare provider if they experience symptoms of hypotension.
- Advise about the risk of hypoglycemia when Labetolol is given to patients who are fasting or who are vomiting. Inform patient to notify their healthcare provider if they experience symptoms of hypoglycemia. [See Warnings and Precautions (5.6)].
Rx Only
Distributed by
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised: April 2023
PIN539-WES/4
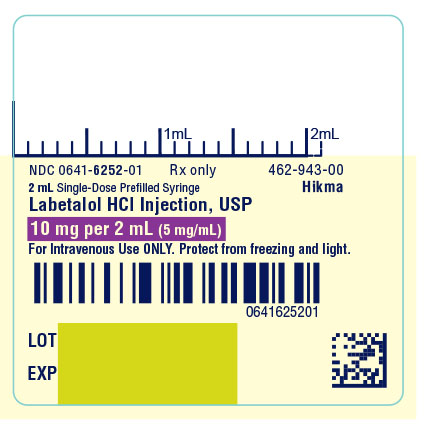
PRINCIPAL DISPLAY PANEL
NDC 0641-6252-01 Rx only
2 mL Single-Dose Prefilled Syringe
Labetalol HCl Injection, USP
10 mg per 2 mL (5 mg/mL)
For Intravenous Use ONLY. Protect from freezing and light.
NDC 0641-6252-10 Rx only
Labetalol HCl Injection, USP
10 mg per 2 mL (5 mg/mL)
For Intravenous Use ONLY.
Preservative Free
10 x 2 mL Single-Dose Prefilled Syringes
Discard unused portion

| LABETALOL HYDROCHLORIDE
labetalol hydrochloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (946499746) |