0.9% SODIUM CHLORIDE injection, solution
0.9% Sodium Chloride by
Drug Labeling and Warnings
0.9% Sodium Chloride by is a Animal medication manufactured, distributed, or labeled by WG Critical Care, LLC, Infomed Fluids SrL. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
0.9% Sodium Chlorideis a sterile, nonpyrogenic solution for fluid replenishment in single dose containers for intravenous administration. Discard unused portion. It contains no antimicrobial agents.
COMPOSITION:
Each 100 mL of sterile aqueous solution contains: Sodium Chloride 900 mg, Water for Injection 100 mL
Milliequivalents per liter
Cations: Sodium 154 mEq/L
Anions: Chloride 154 mEq/L
Total osmolarity is 308 milliosmoles per liter. pH 4.5 to 7.0.
- INDICATIONS
-
WARNINGS
0.9% Sodium Chloride, should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
The intravenous administration of 0.9% Sodium Chloride can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, over-hydration, congested states, or pulmonary edema. The risk of dilutive states is inversely proportional to the electrolyte concentration of the injections. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of the injections.
In patients with diminished renal function, administration of 0.9% Sodium Chloride may result in sodium retention.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of 0.9% Sodium Chloride Intravenous Infusion to patients receiving corticosteroids or corticotrophin.
Do not administer unless solution is clear and seal is intact.
-
DOSAGE AND ADMINISTRATION
As directed by a veterinarian. Dosage is dependent upon the age, weight and clinical condition of the patient, as well as laboratory determinations.
Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration whenever solution and container permit.
All injections in plastic containers are intended for intravenous administration using sterile equipment.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. If, in the informed judgement of the veterinarian, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced. Do not store solutions containing additives.
-
PACKAGING
1000 mL bag with clear overwrap: NDC: 44567-970-10
3000 mL bag with clear overwrap: NDC: 44567-970-30
5000 mL bag with clear overwrap: NDC: 44567-970-50
STORAGE:
Store below 25°C (77°F).
Keep out of reach of children.
Directions for Use of Plastic Container:
To Open
Tear overwrap at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired. If supplemental medication is desired, follow directions below:
Preparation for Administration
(Use Aseptic Technique)
1. Close flow control clamp of administration set.
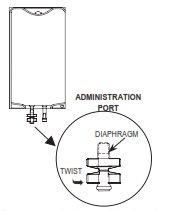
2. Twist off the protector cap from the administration port of the bags.
3. Insert the spike, a slight resistance should be felt as the port membrane is broken.
Use a twisting motion during insertion. Insert the spike until the shoulder of the spike is level with the port.
NOTE: See full directions on administration set packaging.
4. Suspend container from hanger.
5. Squeeze and release drip chamber to establish proper fluid level in chamber.
6. Open flow control clamp and clear air from set. Close clamp.
7. Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
8. Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
To Add Medication
WARNING: Additives may be incompatible.
To add medication before solution administration
1. Prepare medication site.
2. Using syringe with 0.63mm to 0.80mm needle, puncture medication port and inject.
3. Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To add medication during solution administration
1. Close the clamp on the administration set.
2. Prepare medication site.
3. Using syringe with 0.63mm to 0.80mm needle, puncture medication port and inject.
4. Remove container from IV pole and/or turn to an upright position.
5. Evacuate both ports by squeezing them while container is in the upright position.
6. Mix solution and medication thoroughly.
7. Return container to in use position and continue administration.
CAUTION:
Federal law (USA) restricts this drug to use by or on the order of a licensed veterinarian.
Manufactured for:
WG Critical Care,LLC
Paramus, NJ 07652
Manufactured by:
INFOMED FLUIDS S.R.L., Bucharest, Romania
Revised March 2016
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
0.9% SODIUM CHLORIDE
0.9% sodium chloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 44567-970 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44567-970-10 1000 mL in 1 BAG 2 NDC: 44567-970-30 3000 mL in 1 BAG 3 NDC: 44567-970-50 5000 mL in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/05/2018 Labeler - WG Critical Care, LLC (829274633) Registrant - WG Critical Care, LLC (829274633) Establishment Name Address ID/FEI Business Operations Infomed Fluids SrL 681561866 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.