DOOOD Liquid Foundation Neutral with Broad Spectrum SPF50 by Guangzhou Meixi Biotechnology Co., Ltd. 84167-002 Completed
DOOOD Liquid Foundation Neutral with Broad Spectrum SPF50 by
Drug Labeling and Warnings
DOOOD Liquid Foundation Neutral with Broad Spectrum SPF50 by is a Otc medication manufactured, distributed, or labeled by Guangzhou Meixi Biotechnology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
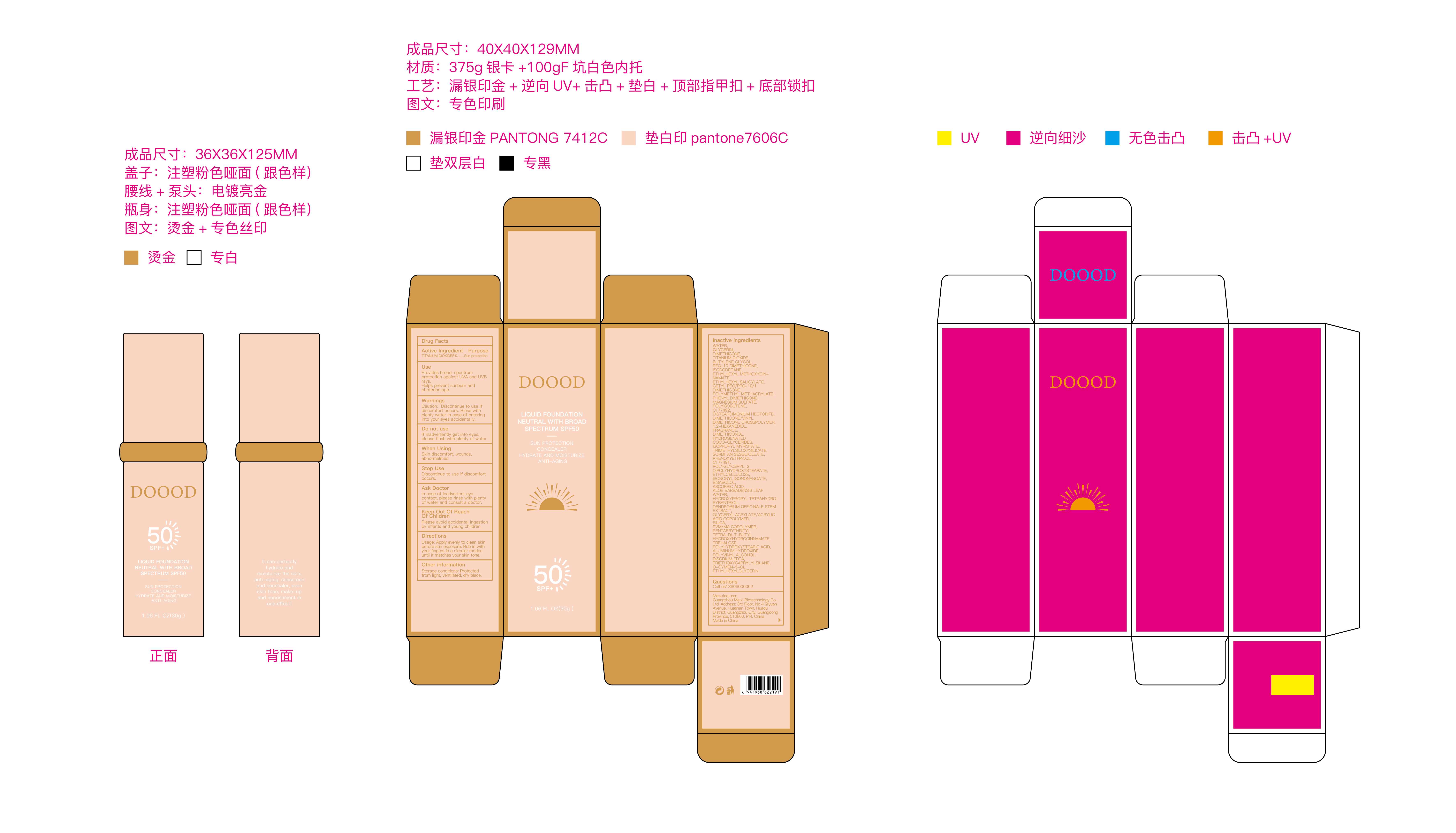
DOOOD LIQUID FOUNDATION NEUTRAL WITH BROAD SPECTRUM SPF50- liquid foundation neutral with broad spectrum spf50 cream
Guangzhou Meixi Biotechnology Co., Ltd.
----------
84167-002 Completed
Use
Provides broad-spectrum protection against UVA and UVB rays.
Helps prevent sunburn and photodamage.
Warnings
Caution: Discontinue to use if discomfort occurs. Rinse with plenty water in case of entering into your eyes accidentally.
Ask Doctor
In case of inadvertent eye contact, please rinse with plenty of water and consult a doctor.
Directions
Usage: Apply evenly to clean skin before sun exposure. Rub in with your fingers in a circular motion until it matches your skin tone.
Inactive ingredients
WATER
GLYCERIN
DIMETHICONE,
BUTYLENE GLYCOL
PEG-10 DIMETHICONE
ISODODECANE
ETHYLHEXYL METHOXYCINNAMATE
ETHYLHEXYL SALICYLATE
CETYL PEG/PPG-10/1 DIMETHICONE
POLYMETHYL METHACRYLATE
PHENYL DIMETHICONE
MAGNESIUM SULFATE
POLYISOBUTENE
CI 77492
DISTEARDIMONIUM HECTORITE
DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER
1,2-HEXANEDIOL
FRAGRANCE
DIMETHICONOL
HYDROGENATED COCO-GLYCERIDES
ISOPROPYL MYRISTATE
TRIMETHYLSILOXYSILICATE
SORBITAN SESQUIOLEATE
PHENOXYETHANOL
CI 77491
POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE
ETHYLCELLULOSE
ISONONYL ISONONANOATE
BISABOLOL
ASCORBIC ACID
ALOE BARBADENSIS LEAF WATER
HYDROXYPROPYL TETRAHYDROPYRANTRIOL
DENDROBIUM OFFICINALE STEM EXTRACT
GLYCERYL ACRYLATE/ACRYLIC ACID COPOLYMER
SILICA
PVM/MA COPOLYMER
PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE
TREHALOSE
POLYHYDROXYSTEARIC ACID
ALUMINUM HYDROXIDE
POLYVINYL ALCOHOL
DISODIUM EDTA
TRIETHOXYCAPRYLYLSILANE
O-CYMEN-5-OL
ETHYLHEXYLGLYCERIN
| DOOOD LIQUID FOUNDATION NEUTRAL WITH BROAD SPECTRUM SPF50
liquid foundation neutral with broad spectrum spf50 cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guangzhou Meixi Biotechnology Co., Ltd. (625724571) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Meixi Biotechnology Co., Ltd. | 625724571 | manufacture(84167-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.