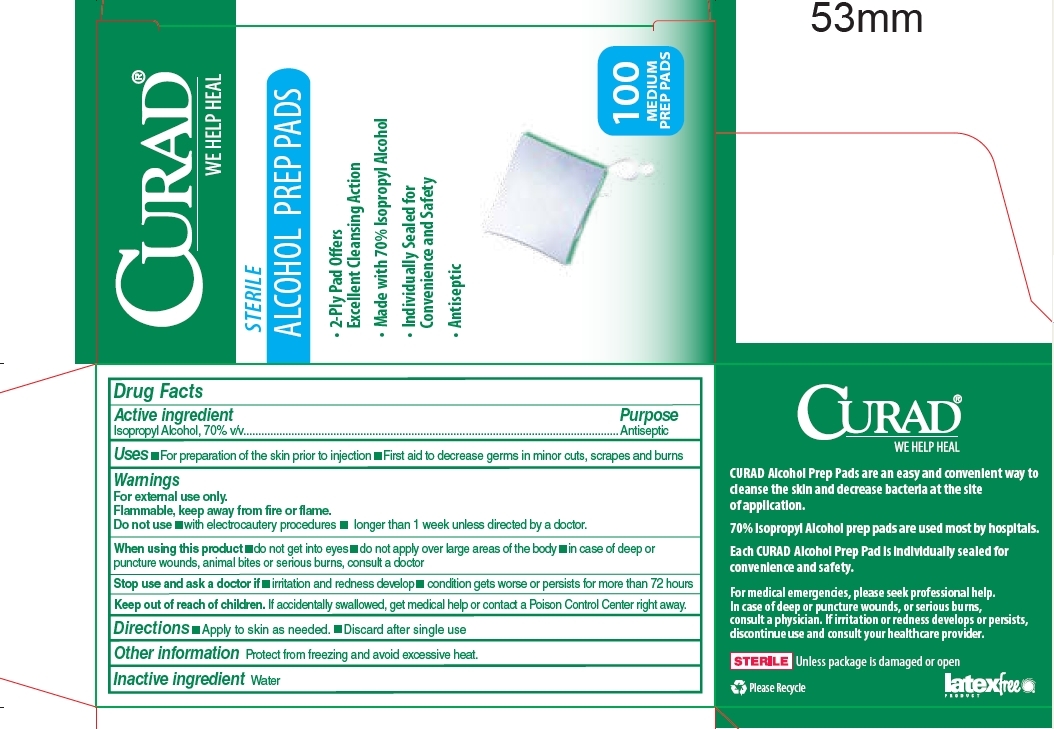
827 Curad Steril Alcohol Prep Pads
Curad Alcohol Prep Pads by
Drug Labeling and Warnings
Curad Alcohol Prep Pads by is a Otc medication manufactured, distributed, or labeled by Medline Industries, LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CURAD ALCOHOL PREP PADS MEDIUM- isopropyl alcohol swab
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
827 Curad Steril Alcohol Prep Pads
Uses
- For preparation of the skin prior to injection
- First aid to decrease germs in minor cuts, scrapes and burns
Warnings
For external use only.
Flammable, keep away from fire or flame.
When using this product
- do not get into eyes
- do not apply over large areas of the body
- in case of deep or puncture wounds, animal bites or serious burns, consult a doctor
| CURAD ALCOHOL PREP PADS
MEDIUM
isopropyl alcohol swab |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shandong Haiyan Medical Manufacture Co., Ltd. | 421283439 | manufacture(53329-827) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lights Medical Manufacture Co., Ltd. | 529128649 | manufacture(53329-827) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Phoenix Innovative Healthcare Manufacturers Private Limited | 650687176 | manufacture(53329-827) | |
Revised: 12/2019
Document Id: 9a9da707-6300-11c8-e053-2995a90a3fcc
Set id: 1ce3872e-2307-4a38-bd7d-7eb2b226cf3c
Version: 7
Effective Time: 20191226
Medline Industries, Inc.