ARNICA- arnica montana pellet
Arnica by
Drug Labeling and Warnings
Arnica by is a Homeopathic medication manufactured, distributed, or labeled by Laboratoires Boiron, Boiron Inc., Boiron. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
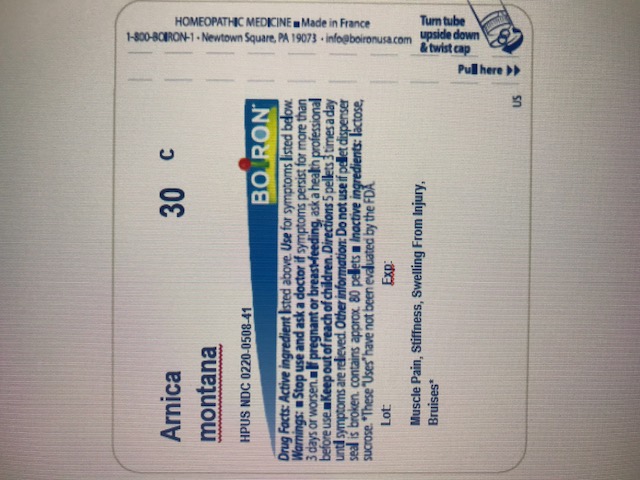
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DO NOT USE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
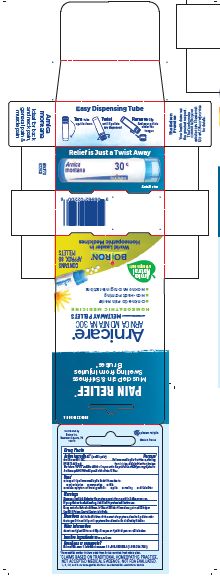
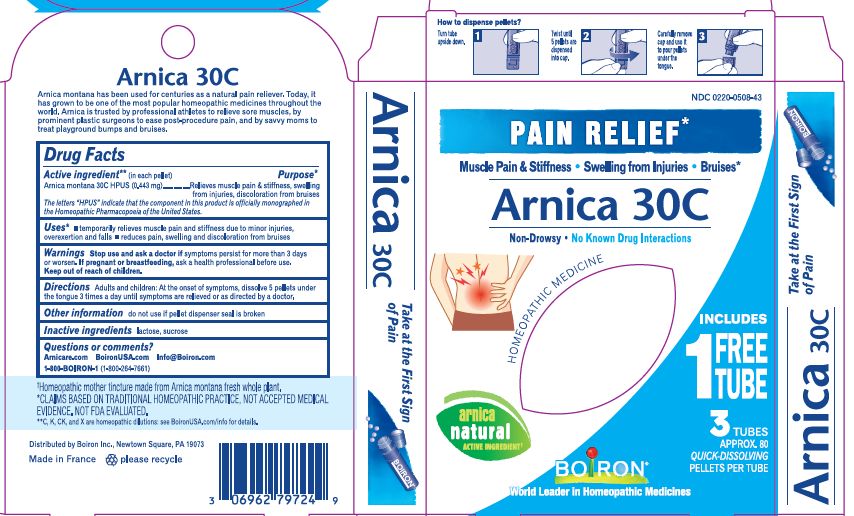
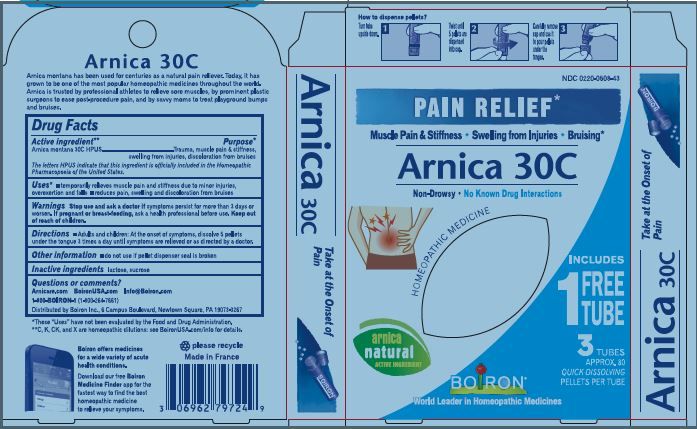
Pain Relief"
Non Drowsy, No Known Drug Interactions, On-the-Go Pain Relief, Non-Habit Forming
How to dispense pellets? Turn tube upside down. Twist until 5 pellets are dispensed into cap. Carefully remove the cap and use it to pour pellets under the tongue.
3 Tubes approx 80 quick dissolving pellets per tube
ɨThe letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
*Claims are based on traditional homeopathic practice, not accepted medical evidence, not FDA evaluated
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
Arnica Montana has been used for centuries as a natural pain reliever. Today, it has grown to be one of the most popular homeopathic medicines throughout the world. Arnica is trusted by professional athletes to relieve sore muscles, by prominent plastic surgeons to ease post-procedure pain, and by savvy moms to treat playground bumps and bruises.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICA
arnica montana pelletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0220-0508 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) LACTOSE (UNII: J2B2A4N98G) Product Characteristics Color white Score no score Shape ROUND (Pellet) Size 4mm Flavor Imprint Code ; Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0220-0508-43 3 in 1 PACKAGE 05/01/2009 1 NDC: 0220-0508-41 80 in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0220-0508-41 80 in 1 TUBE; Type 0: Not a Combination Product 05/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/01/2009 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-0508)
Trademark Results [Arnica]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARNICA 87317515 not registered Dead/Abandoned |
NICOLE STERN 2017-01-30 |
 ARNICA 77399483 3563474 Dead/Cancelled |
SING, KENNY 2008-02-18 |
 ARNICA 75088650 2184529 Dead/Cancelled |
Lozhkin, Igor 1996-04-11 |
 ARNICA 73366355 not registered Dead/Abandoned |
ARNICA INTERNATIONAL S. A. 1982-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.