FESOTERODINE FUMARATE tablet, extended release
Fesoterodine Fumarate by
Drug Labeling and Warnings
Fesoterodine Fumarate by is a Prescription medication manufactured, distributed, or labeled by Amneal Pharmaceuticals LLC, Amneal Pharmaceuticals of New York, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS.
FESOTERODINE fumarate extended-release tablets, for oral use
Initial U.S. Approval: 2008INDICATIONS AND USAGE
Fesoterodine fumarate extended-release tablets are indicated for the treatment of:
- Overactive bladder (OAB) in adults with symptoms of urge urinary incontinence, urgency, and frequency. (1.1)
DOSAGE AND ADMINISTRATION
- OAB in Adults: The recommended starting dosage is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of 8 mg once daily. (2.1)
- Adult Patients with Renal Impairment: Refer to the full prescribing information for recommended dosage. (2.3)
- Dosage Modifications Due to Strong CYP3A4 Inhibitors: Refer to the full prescribing information for recommended dosage. (2.5)
- Administration: Swallow whole with liquid. Do not chew, divide, or crush. Take with or without food. (2.6)
DOSAGE FORMS AND STRENGTHS
Extended-release tablets: 4 mg and 8 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Angioedema: Promptly discontinue fesoterodine fumarate and provide appropriate therapy. (5.1)
- Urinary Retention: Fesoterodine fumarate is not recommended in patients with clinically significant bladder outlet obstruction because of the risk of urinary retention. (5.2)
- Decreased Gastrointestinal Motility: Fesoterodine fumarate is not recommended for use in patients with decreased gastrointestinal motility, such as those with severe constipation. (5.3)
- Worsening of Narrow-Angle Glaucoma: Use fesoterodine fumarate with caution in patients being treated for narrow-angle glaucoma. (5.4)
- Central Nervous System Effects: Somnolence has been reported with fesoterodine fumarate. Advise patients not to drive or operate heavy machinery until they know how fesoterodine fumarate affects them. (5.5)
- Worsening of Myasthenia Gravis Symptoms: Use fesoterodine fumarate with caution in patients with myasthenia gravis. (5.6)
ADVERSE REACTIONS
- Most frequently reported adverse events with fesoterodine fumarate in adult patients with OAB (≥4%) were: dry mouth (placebo, 7%; fesoterodine fumarate 4 mg, 19%; fesoterodine fumarate 8 mg, 35%) and constipation (placebo, 2%; fesoterodine fumarate 4 mg, 4%; fesoterodine fumarate 8 mg, 6%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Overactive Bladder
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients With OAB
2.3 Recommended Dosage in Adult Patients With Renal Impairment
2.5 Fesoterodine Fumarate Extended-Release Tablets Dosage Modifications Due to Strong CYP3A4 Inhibitors
2.6 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
5.2 Urinary Retention in Adult Patients With Bladder Outlet Obstruction
5.3 Decreased Gastrointestinal Motility
5.4 Worsening of Narrow-Angle Glaucoma
5.5 Central Nervous System Effects
5.6 Worsening of Myasthenia Gravis Symptoms
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antimuscarinic Drugs
7.2 CYP3A4 Inhibitors
7.3 CYP3A4 Inducers
7.4 CYP2D6 Inhibitors
7.5 Drugs Metabolized by Cytochrome P450
7.6 Oral Contraceptives
7.7 Warfarin
7.8 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Overactive Bladder
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Adult Overactive Bladder
Fesoterodine fumarate extended-release tablets are indicated for the treatment of overactive bladder (OAB) in adults with symptoms of urge urinary incontinence, urgency, and frequency.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients With OAB
The recommended starting dosage of fesoterodine fumarate extended-release tablets in adults is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of fesoterodine fumarate extended-release tablets 8 mg once daily. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Recommended Dosage in Adult Patients With Renal Impairment
The recommended dosage of fesoterodine fumarate extended-release tablets in adult patients with renal impairment is described in Table 1 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 1: Fesoterodine Fumarate Extended-Release Tablets Recommended Dose in Adult Patients With Renal Impairment (Administered Orally Once Daily)
Estimated Creatinine Clearance1
Recommended Dose
CLcr 30 to 89 mL/min
8 mg
CLcr 15 to 29 mL/min
4 mg
CLcr <15 mL/min
4 mg
1 Calculate CLcr using the Cockcroft-Gault formula
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.5 Fesoterodine Fumarate Extended-Release Tablets Dosage Modifications Due to Strong CYP3A4 Inhibitors
Adult Patients with OAB
The maximum recommended dosage is fesoterodine fumarate extended-release tablets 4 mg orally once daily in adult patients taking strong CYP3A4 inhibitors [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.6 Administration Instructions
Swallow fesoterodine fumarate extended-release tablets whole with liquid. Do not chew, divide, or crush. Take with or without food [see Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Fesoterodine fumarate extended-release tablets are contraindicated in patients with any of the following:
- known or suspected hypersensitivity to fesoterodine fumarate extended-release tablets or any of its ingredients, or to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules [see Clinical Pharmacology (12.1)]. Reactions have included angioedema [see Warnings and Precautions (5.1)].
- urinary retention [see Warnings and Precautions (5.2)]
- gastric retention [see Warnings and Precautions (5.3)]
- uncontrolled narrow-angle glaucoma [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
Angioedema of the face, lips, tongue, and/or larynx has been reported with fesoterodine fumarate. In some cases, angioedema occurred after the first dose; however, cases have been reported to occur hours after the first dose or after multiple doses. Angioedema associated with upper airway swelling may be life-threatening.
Fesoterodine fumarate is contraindicated in patients with a known or suspected hypersensitivity to fesoterodine fumarate extended-release tablets or any of its ingredients [see Contraindications (4)]. If involvement of the tongue, hypopharynx, or larynx occurs, fesoterodine should be promptly discontinued and appropriate therapy and/or measures to ensure a patent airway should be promptly provided.
5.2 Urinary Retention in Adult Patients With Bladder Outlet Obstruction
The use of fesoterodine fumarate, like other antimuscarinic drugs, in patients with clinically significant bladder outlet obstruction, including patients with urinary retention, may result in further urinary retention and kidney injury. The use of fesoterodine fumarate is not recommended in patients with clinically significant bladder outlet obstruction, and is contraindicated in patients with urinary retention [see Contraindications (4) and Adverse Reactions (6.1)].
5.3 Decreased Gastrointestinal Motility
Fesoterodine fumarate is associated with decreased gastric motility. Fesoterodine fumarate is contraindicated in patients with gastric retention [see Contraindications (4)]. The use of fesoterodine fumarate is not recommended in patients with decreased gastrointestinal motility, such as those with severe constipation.
5.4 Worsening of Narrow-Angle Glaucoma
Fesoterodine fumarate can worsen controlled narrow-angle glaucoma. Fesoterodine fumarate is contraindicated in patients with uncontrolled narrow-angle glaucoma [see Contraindications (4)]. Fesoterodine fumarate should be used with caution in patients being treated for narrow-angle glaucoma.
5.5 Central Nervous System Effects
Fesoterodine fumarate is associated with anticholinergic central nervous system (CNS) adverse reactions [see Adverse Reactions (6.1)]. A variety of CNS anticholinergic effects have been reported, including headache, dizziness, and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how fesoterodine fumarate affects them. If a patient experiences anticholinergic CNS effects, fesoterodine fumarate dose reduction or discontinuation should be considered.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Angioedema [see Warnings and Precautions (5.1)]
- Urinary Retention [see Warnings and Precautions (5.2)]
- Decreased Gastrointestinal Motility [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Overactive Bladder (OAB)
The safety of fesoterodine fumarate was evaluated in Phase 2 and 3 controlled trials in a total of 2,859 patients with overactive bladder, of which 2,288 were treated with fesoterodine fumarate. Of this total, 782 received fesoterodine fumarate 4 mg/day, and 785 received fesoterodine fumarate 8 mg/day with treatment periods of 8- or 12-weeks. Approximately 80% of these patients had greater than 10-weeks of exposure to fesoterodine fumarate in these trials.
A total of 1,964 patients participated in two 12-week, Phase 3 efficacy and safety studies and subsequent open-label extension studies. In these two studies combined, 554 patients received fesoterodine fumarate 4 mg/day and 566 patients received fesoterodine fumarate 8 mg/day.
In Phase 2 and 3 placebo-controlled trials combined, the incidences of serious adverse events in patients receiving placebo, fesoterodine fumarate 4 mg, and fesoterodine fumarate 8 mg were 1.9%, 3.5%, and 2.9%, respectively. All serious adverse events were judged to be not related or unlikely to be related to study medication by the investigator, except for four patients receiving fesoterodine fumarate who reported one serious adverse reaction each: angina, chest pain, gastroenteritis, and QT prolongation on ECG.
The most commonly reported adverse event in patients treated with fesoterodine fumarate was dry mouth. The incidence of dry mouth was higher in those taking 8 mg/day (35%) and in those taking 4 mg/day (19%), as compared to placebo (7%). Dry mouth led to discontinuation in 0.4%, 0.4%, and 0.8% of patients receiving placebo, fesoterodine fumarate 4 mg, and fesoterodine fumarate 8 mg, respectively. For those patients who reported dry mouth, most had their first occurrence of the event within the first month of treatment.
The second most commonly reported adverse event was constipation. The incidence of constipation was 2% in those taking placebo, 4% in those taking 4 mg/day, and 6% in those taking 8 mg/day.
Table 4 lists adverse events, regardless of causality, that were reported in the combined Phase 3, randomized, placebo-controlled trials at an incidence greater than placebo and in 1% or more of patients treated with fesoterodine fumarate 4 mg or 8 mg once daily for up to 12-weeks.
Table 4: Adverse Events With an Incidence Exceeding the Placebo Rate and Reported by ≥1% of Patients From Double-Blind, Placebo-Controlled Phase 3 Trials of 12-weeks Treatment Duration
System organ class/Preferred term
Placebo
N=554
%Fesoterodine fumarate
4 mg/day
N=554
%Fesoterodine fumarate
8 mg/day
N=566
%Gastrointestinal disorders
Dry mouth
7.0
18.8
34.6
Constipation
2.0
4.2
6.0
Dyspepsia
0.5
1.6
2.3
Nausea
1.3
0.7
1.9
Abdominal pain upper
0.5
1.1
0.5
Infections
Urinary tract infection
3.1
3.2
4.2
Upper respiratory tract infection
2.2
2.5
1.8
Eye disorders
Dry eyes
0
1.4
3.7
Renal and urinary disorders
Dysuria
0.7
1.3
1.6
Urinary retention
0.2
1.1
1.4
Respiratory disorders
Cough
0.5
1.6
0.9
Dry throat
0.4
0.9
2.3
General disorders
Edema peripheral
0.7
0.7
1.2
Musculoskeletal disorders
Back pain
0.4
2.0
0.9
Psychiatric disorders
Insomnia
0.5
1.3
0.4
Investigations
ALT increased
0.9
0.5
1.2
GGT increased
0.4
0.4
1.2
Skin disorders
Rash
0.5
0.7
1.1
ALT = alanine aminotransferase; GGT = gamma glutamyltransferase
Patients also received fesoterodine fumarate for up to three years in open-label extension phases of one Phase 2 and two Phase 3 controlled trials. In all open-label trials combined, 857, 701, 529, and 105 patients received fesoterodine fumarate for at least 6 months, 1 year, 2 years, and 3 years, respectively. The adverse events observed during long-term, open-label studies were similar to those observed in the 12-week, placebo-controlled studies, and included dry mouth, constipation, dry eyes, dyspepsia, and abdominal pain. Similar to the controlled studies, most adverse events of dry mouth and constipation were mild to moderate in intensity. Serious adverse events, judged to be at least possibly related to study medication by the investigator and reported more than once during the open-label treatment period of up to 3 years, included urinary retention (3 cases), diverticulitis (3 cases), constipation (2 cases), irritable bowel syndrome (2 cases), and electrocardiogram QT corrected interval prolongation (2 cases).
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fesoterodine fumarate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: Palpitations
Central nervous system disorders: Dizziness, headache, somnolence
Eye disorders: Blurred vision
Gastrointestinal disorders: Hypoaesthesia oral
General disorders and administrative site conditions: Hypersensitivity reactions, including angioedema with airway obstruction, face edema
Psychiatric disorders: Confusional state
Skin and subcutaneous tissue disorders: Urticaria, pruritus
-
7 DRUG INTERACTIONS
7.1 Antimuscarinic Drugs
Co-administration of fesoterodine fumarate with other antimuscarinic agents that produce dry mouth, constipation, urinary retention, and other anticholinergic pharmacological effects may increase the frequency and/or severity of such effects. Anticholinergic agents may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility.
7.2 CYP3A4 Inhibitors
Doses of fesoterodine fumarate greater than 4 mg are not recommended in adult patients taking strong CYP3A4 inhibitors, such as ketoconazole, itraconazole, and clarithromycin [see Dosage and Administration (2.5)].
In a study in adults, co-administration of the strong CYP3A4 inhibitor ketoconazole with fesoterodine led to approximately a doubling of the maximum concentration (Cmax) and area under the concentration versus time curve (AUC) of 5-hydroxymethyl tolterodine (5-HMT), the active metabolite of fesoterodine. Compared with CYP2D6 extensive metabolizers not taking ketoconazole, further increases in the exposure to 5-HMT were observed in subjects who were CYP2D6 poor metabolizers taking ketoconazole [see Clinical Pharmacology (12.3)].
There is no clinically relevant effect of moderate CYP3A4 inhibitors on the pharmacokinetics of fesoterodine. Following blockade of CYP3A4 by co-administration of the moderate CYP3A4 inhibitor fluconazole 200 mg twice a day for 2 days, the average (90% confidence interval) increase in Cmax and AUC of the active metabolite of fesoterodine was approximately 19% (11% to 28%) and 27% (18% to 36%) respectively. No dosing adjustments are recommended in the presence of moderate CYP3A4 inhibitors (e.g., erythromycin, fluconazole, diltiazem, verapamil and grapefruit juice).
The effect of weak CYP3A4 inhibitors (e.g. cimetidine) was not examined; it is not expected to be in excess of the effect of moderate inhibitors [see Clinical Pharmacology (12.3)].
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
7.3 CYP3A4 Inducers
No dosing adjustments are recommended in the presence of CYP3A4 inducers, such as rifampin and carbamazepine. Following induction of CYP3A4 by co-administration of rifampin 600 mg once a day, Cmax and AUC of the active metabolite of fesoterodine decreased by approximately 70% and 75%, respectively, after oral administration of fesoterodine fumarate 8 mg. The terminal half-life of the active metabolite was not changed.
7.4 CYP2D6 Inhibitors
The interaction with CYP2D6 inhibitors was not tested clinically. In poor metabolizers for CYP2D6, representing a maximum CYP2D6 inhibition, Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively.
No dosing adjustments are recommended in the presence of CYP2D6 inhibitors.
7.5 Drugs Metabolized by Cytochrome P450
In vitro data indicate that at therapeutic concentrations, the active metabolite of fesoterodine does not have the potential to inhibit or induce Cytochrome P450 enzyme systems [see Clinical Pharmacology (12.3)].
7.6 Oral Contraceptives
In the presence of fesoterodine, there are no clinically significant changes in the plasma concentrations of combined oral contraceptives containing ethinyl estradiol and levonorgestrel [see Clinical Pharmacology (12.3)].
7.7 Warfarin
A clinical study has shown that fesoterodine 8 mg once daily has no significant effect on the pharmacokinetics or the anticoagulant activity (PT/INR) of warfarin 25 mg. Standard therapeutic monitoring for warfarin should be continued [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with the use of fesoterodine fumarate in pregnant women and adolescents to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of fesoterodine to pregnant mice and rabbits during organogenesis resulted in fetotoxicity at maternal exposures that were 6 and 3 times respectively, the maximum recommended human dose (MRHD) of 8 mg/day, based on AUC (see Data). The background risk of major birth defects and miscarriage for the indicated population are unknown. However, in the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No dose-related teratogenicity was observed in reproduction studies performed in mice and rabbits. In mice at 6 to 27 times the expected exposure at the maximum recommended human dose (MRHD) of 8 mg based on AUC (75 mg/kg/day, oral), increased resorptions and decreased live fetuses were observed. One fetus with cleft palate was observed at each dose (15, 45, and 75 mg/kg/day), at an incidence within the background historical range. In rabbits treated at 3 to 11 times the MRHD (27 mg/kg/day, oral), incompletely ossified sternebrae (retardation of bone development) and reduced survival were observed in fetuses. In rabbits at 9 to 11 times the MRHD (4.5 mg/kg/day, subcutaneous), maternal toxicity and incompletely ossified sternebrae were observed in fetuses (at an incidence within the background historical range). In rabbits at 3 times the MRHD (1.5 mg/kg/day, subcutaneous), decreased maternal food consumption in the absence of any fetal effects was observed. Oral administration of 30 mg/kg/day fesoterodine to mice in a pre- and post-natal development study resulted in decreased body weight of the dams and delayed ear opening of the pups. No effects were noted on mating and reproduction of the F1 dams or on the F2 offspring.
8.2 Lactation
Risk Summary
There is no information on the presence of fesoterodine in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for fesoterodine fumarate and any potential adverse effects on the breastfed child from fesoterodine fumarate or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of fesoterodine fumarate have not been established in pediatric patients younger than 6 years of age or weighing 25 kg or less.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
No dose adjustment is recommended for the elderly. The pharmacokinetics of fesoterodine are not significantly influenced by age.
Of the 1,567 patients who received fesoterodine fumarate 4 mg or 8 mg orally once daily in Phase 2 and 3, placebo-controlled, efficacy and safety studies for OAB, 515 (33%) were 65 years of age or older, and 140 (9%) were 75 years of age or older. No overall difference in effectiveness was observed between patients younger than 65 years of age and those 65 years of age or older in these studies. However, the incidence of antimuscarinic adverse reactions, including dry mouth, constipation, dyspepsia, increase in residual urine, dizziness (8 mg only) and urinary tract infection, was higher in patients 75 years of age and older as compared to younger patients [see Clinical Studies (14.1) and Adverse Reactions (6)].
8.6 Renal Impairment
In adult patients with severe renal impairment (CLCR < 30 mL/min), Cmax and AUC are increased 2.0- and 2.3-fold, respectively. Doses of fesoterodine fumarate greater than 4 mg are not recommended in adult patients with severe renal impairment. In patients with mild or moderate renal impairment (CLCR ranging from 30 to 80 mL/min), Cmax and AUC of the active metabolite are increased up to 1.5- and 1.8-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate renal impairment [see Clinical Pharmacology (12.3) and Dosage and Administration (2.3)].
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.7 Hepatic Impairment
Patients with severe hepatic impairment (Child-Pugh C) have not been studied; therefore, fesoterodine fumarate is not recommended for use in these patients. In patients with moderate (Child-Pugh B) hepatic impairment, Cmax and AUC of the active metabolite are increased 1.4- and 2.1-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate hepatic impairment [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Fesoterodine fumarate extended-release tablets contain fesoterodine fumarate. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a muscarinic receptor antagonist.
Chemically, fesoterodine fumarate is designated as isobutyric acid 2-((R)-3-diisopropylammonium-1-phenylpropyl)-4-(hydroxymethyl) phenyl ester hydrogen fumarate. The empirical formula is C30H41NO7 and its molecular weight is 527.66. The structural formula is:
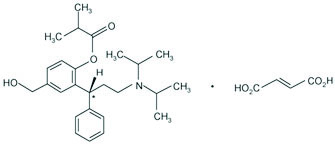
The asterisk (*) indicates the chiral carbon.
Fesoterodine fumarate is a white to off-white powder, which is freely soluble in water. Each fesoterodine fumarate extended-release tablet contains either 4 mg or 8 mg of fesoterodine fumarate and the following inactive ingredients: FD&C Blue No. 2, fructose granular, glyceryl behenate, hypromellose, lactose monohydrate, lecithin (soya), maize starch, polyvinyl alcohol, talc, titanium dioxide and xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fesoterodine is a competitive muscarinic receptor antagonist. After oral administration, fesoterodine is rapidly and extensively hydrolyzed by nonspecific esterases to its active metabolite, 5-hydroxymethyl tolterodine, which is responsible for the antimuscarinic activity of fesoterodine.
Muscarinic receptors play a role in contractions of urinary bladder smooth muscle. Inhibition of these receptors in the bladder is presumed to be the mechanism by which fesoterodine produces its effects.
12.2 Pharmacodynamics
In a urodynamic study involving patients with involuntary detrusor contractions, the effects after the administration of fesoterodine on the volume at first detrusor contraction and bladder capacity were assessed. Administration of fesoterodine increased the volume at first detrusor contraction and bladder capacity in a dose-dependent manner. These findings are consistent with an antimuscarinic effect on the bladder.
Cardiac Electrophysiology
The effect of fesoterodine 4 mg and 28 mg on the QT interval was evaluated in a double-blind, randomized, placebo- and positive-controlled (moxifloxacin 400 mg once a day) parallel trial with once-daily treatment over a period of 3 days in 261 male and female subjects aged 44 to 65 years. Electrocardiographic parameters were measured over a 24-hour period at pre-dose, after the first administration, and after the third administration of study medication. Fesoterodine 28 mg was chosen because this dose, when administered to CYP2D6 extensive metabolizers, results in an exposure to the active metabolite that is similar to the exposure in a CYP2D6 poor metabolizer receiving fesoterodine 8 mg together with CYP3A4 blockade. Corrected QT intervals (QTc) were calculated using Fridericia’s correction and a linear individual correction method. Analyses of 24-hour average QTc, time-matched baseline-corrected QTc, and time-matched placebo-subtracted QTc intervals indicate that fesoterodine at doses of 4 and 28 mg/day did not prolong the QT interval. The sensitivity of the study was confirmed by positive QTc prolongation by moxifloxacin.
In this study, conducted in subjects aged 44 to 65 years, fesoterodine fumarate was associated with an increase in heart rate that correlates with increasing dose. When compared to placebo, the mean increase in heart rate associated with fesoterodine 4 mg/day and fesoterodine 28 mg/day was 3 beats/minute and 11 beats/minute, respectively.
In the two, phase 3, placebo-controlled studies in adult patients with overactive bladder, the mean increases in heart rate compared to placebo were 3 to 4 beats/minute in the fesoterodine 4 mg/day group and 3 to 5 beats/minute in the fesoterodine 8 mg/day group.
12.3 Pharmacokinetics
Absorption
After oral administration, fesoterodine is well absorbed. Due to rapid and extensive hydrolysis by nonspecific esterases to its active metabolite 5-hydroxymethyl tolterodine, fesoterodine cannot be detected in plasma. Bioavailability of the active metabolite is 52%. After single or multiple-dose oral administration of fesoterodine in doses from 4 mg to 28 mg, plasma concentrations of the active metabolite are proportional to the dose. Maximum plasma levels are reached after approximately 5 hours. No accumulation occurs after multiple-dose administration.
A Summary of pharmacokinetic parameters for the active metabolite after a single dose of fesoterodine fumarate 4 mg and 8 mg in extensive and poor metabolizers of CYP2D6 is provided in Table 8.
Table 8: Summary of Geometric Mean [CV] Pharmacokinetic Parameters for the Active Metabolite After a Single Dose of fesoterodine fumarate 4 mg and 8 mg
in Extensive and Poor CYP2D6 MetabolizersFesoterodine fumarate 4 mg
Fesoterodine fumarate 8 mg
Parameter
EM (N=16)
PM (N=8)
EM (N=16)
PM (N=8)
Cmax (ng/mL)
1.89 [43%]
3.45 [54%]
3.98 [28%]
6.90 [39%]
AUC0-tz
(ng*h/mL)21.2 [38%]
40.5 [31%]
45.3 [32%]
88.7 [36%]
tmax (h)a
5 [2 to 6]
5 [5 to 6]
5 [3 to 6]
5 [5 to 6]
t1/2 (h)
7.31 [27%]
7.31 [30%]
8.59 [41%]
7.66 [21%]
EM = extensive CYP2D6 metabolizer, PM = poor CYP2D6 metabolizer, CV = coefficient of variation;
Cmax = maximum plasma concentration, AUC0-tz = area under the concentration time curve from zero up to the last measurable plasma concentration, tmax = time to reach Cmax, t1/2 = terminal half-life
a Data presented as median (range)
Effect of Food
There is no clinically relevant effect of food on the pharmacokinetics of fesoterodine. In a study of the effects of food on the pharmacokinetics of fesoterodine in 16 healthy male volunteers, concomitant food intake increased the active metabolite of fesoterodine AUC by approximately 19% and Cmax by 18% [see Dosage and Administration (2.1)].
Distribution
Plasma protein binding of the active metabolite is low (approximately 50%) and is primarily bound to albumin and alpha-1-acid glycoprotein. The mean steady-state volume of distribution following intravenous infusion of the active metabolite is 169 L.
Metabolism
After oral administration, fesoterodine is rapidly and extensively hydrolyzed to its active metabolite. The active metabolite is further metabolized in the liver to its carboxy, carboxy-N-desisopropyl, and N-desisopropyl metabolites via two major pathways involving CYP2D6 and CYP3A4. None of these metabolites contribute significantly to the antimuscarinic activity of fesoterodine.
Variability in CYP2D6 Metabolism
A subset of individuals (approximately 7% of Caucasians and approximately 2% of African Americans) are poor metabolizers for CYP2D6. Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively, in CYP2D6 poor metabolizers, as compared to extensive metabolizers.
Excretion
Hepatic metabolism and renal excretion contribute significantly to the elimination of the active metabolite. After oral administration of fesoterodine, approximately 70% of the administered dose was recovered in urine as the active metabolite (16%), carboxy metabolite (34%), carboxy-N-desisopropyl metabolite (18%), or N-desisopropyl metabolite (1%), and a smaller amount (7%) was recovered in feces.
The terminal half-life of the active metabolite is approximately 4 hours following an intravenous administration. The apparent terminal half-life following oral administration is approximately 7 hours.
Pharmacokinetics in Specific Populations
Geriatric Patients
Following a single 8 mg oral dose of fesoterodine, the mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in 12 elderly men (mean age 67 years) were 51.8 ± 26.1 h*ng/mL and 3.8 ± 1.7 ng/mL, respectively. In the same study, the mean (±SD) AUC and Cmax in 12 young men (mean age 30 years) were 52.0 ± 31.5 h*ng/mL and 4.1 ± 2.1 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by age [see Use in Specific Populations (8.5)].
Gender
Following a single 8 mg oral dose of fesoterodine, the mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in 12 elderly men (mean age 67 years) were 51.8 ± 26.1 h*ng/mL and 3.8 ± 1.7 ng/mL, respectively. In the same study, the mean (±SD) AUC and Cmax in 12 elderly women (mean age 68 years) were 56.0 ± 28.8 h*ng/mL and 4.6 ± 2.3 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by gender.
Race
The effects of Caucasian or Black race on the pharmacokinetics of fesoterodine were examined in a study of 12 Caucasian and 12 Black African young male volunteers. Each subject received a single oral dose of 8 mg fesoterodine. The mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in Caucasian males were 73.0 ± 27.8 h*ng/mL and 6.1 ± 2.7 ng/mL, respectively. The mean (±SD) AUC and Cmax in Black males were 65.8 ± 23.2 h*ng/mL and 5.5 ± 1.9 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by race.
Renal Impairment
In patients with mild or moderate renal impairment (CLCR ranging from 30 to 80 mL/min), Cmax and AUC of the active metabolite are increased up to 1.5- and 1.8-fold, respectively, as compared to healthy subjects. In patients with severe renal impairment (CLCR < 30 mL/min), Cmax and AUC are increased 2.0- and 2.3-fold, respectively [see Use in Specific Populations (8.6) and Dosage and Administration (2.3)].
Hepatic Impairment
In patients with moderate (Child-Pugh B) hepatic impairment, Cmax and AUC of the active metabolite are increased 1.4- and 2.1-fold, respectively, as compared to healthy subjects.
Subjects with severe hepatic impairment (Child-Pugh C) have not been studied [see Use in Specific Populations (8.7)].
Drug-Drug Interactions
Drugs Metabolized by Cytochrome P450
At therapeutic concentrations, the active metabolite of fesoterodine does not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, 2C19, or 3A4 in vitro [see Drug Interactions (7.5)].
CYP3A4 Inhibitors
Following blockade of CYP3A4 by co-administration of the strong CYP3A4 inhibitor ketoconazole 200 mg twice a day for 5 days, Cmax and AUC of the active metabolite of fesoterodine increased 2.0- and 2.3-fold, respectively, after oral administration of fesoterodine fumarate 8 mg to CYP2D6 extensive metabolizers. In CYP2D6 poor metabolizers, Cmax and AUC of the active metabolite of fesoterodine increased 2.1- and 2.5-fold, respectively, during co-administration of ketoconazole 200 mg twice a day for 5 days. Cmax and AUC were 4.5- and 5.7-fold higher, respectively, in subjects who were CYP2D6 poor metabolizers and taking ketoconazole compared to subjects who were CYP2D6 extensive metabolizers and not taking ketoconazole. In a separate study co-administering fesoterodine with ketoconazole 200 mg once a day for 5 days, the Cmax and AUC values of the active metabolite of fesoterodine were increased 2.2-fold in CYP2D6 extensive metabolizers and 1.5- and 1.9-fold, respectively, in CYP2D6 poor metabolizers. Cmax and AUC were 3.4- and 4.2-fold higher, respectively, in subjects who were CYP2D6 poor metabolizers and taking ketoconazole compared to subjects who were CYP2D6 extensive metabolizers and not taking ketoconazole.
There is no clinically relevant effect of moderate CYP3A4 inhibitors on the pharmacokinetics of fesoterodine. In a drug-drug interaction study evaluating the co-administration of the moderate CYP3A4 inhibitor fluconazole 200 mg twice a day for 2 days, a single 8 mg dose of fesoterodine was administered 1 hour following the first dose of fluconazole on day 1 of the study. The average (90% confidence interval) for the increase in Cmax and AUC of the active metabolite of fesoterodine was approximately 19% (11% to 28%) and 27% (18% to 36%) respectively.
The effect of weak CYP3A4 inhibitors (e.g. cimetidine) was not examined; it is not expected to be in excess of the effect of moderate inhibitors [see Drug Interactions (7.2) and Dosage and Administration (2.3)].
CYP3A4 Inducers
Following induction of CYP3A4 by co-administration of rifampicin 600 mg once a day, Cmax and AUC of the active metabolite of fesoterodine decreased by approximately 70% and 75%, respectively, after oral administration of fesoterodine fumarate 8 mg. The terminal half-life of the active metabolite was not changed.
Induction of CYP3A4 may lead to reduced plasma levels. No dosing adjustments are recommended in the presence of CYP3A4 inducers [see Drug Interactions (7.3)].
CYP2D6 Inhibitors
The interaction with CYP2D6 inhibitors was not studied. In poor metabolizers for CYP2D6, representing a maximum CYP2D6 inhibition, Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively [see Drug Interactions (7.4)].
Oral Contraceptives
Thirty healthy female subjects taking an oral contraceptive containing 0.03 mg ethinyl estradiol and 0.15 mg levonorgestrel were evaluated in a 2-period cross-over study. Each subject was randomized to receive concomitant administration of either placebo or fesoterodine 8 mg once daily on days 1 to 14 of hormone cycle for 2 consecutive cycles. Pharmacokinetics of ethinyl estradiol and levonorgestrel were assessed on day 13 of each cycle. Fesoterodine increased the AUC and Cmax of ethinyl estradiol by 1% to 3% and decreased the AUC and Cmax of levonorgestrel by 11% to 13% [see Drug Interactions (7.6)].
Warfarin
In a cross-over study in 14 healthy male volunteers (18 to 55 years), a single oral dose of warfarin 25 mg was given either alone or on day 3 of once daily dosing for 9 days with fesoterodine 8 mg. Compared to warfarin alone dosing, the Cmax and AUC of S-warfarin were lower by ~ 4 %, while the Cmax and AUC of R-warfarin were lower by approximately 8% and 6% for the co-administration, suggesting absence of a significant pharmacokinetic interaction.
There were no statistically significant changes in the measured pharmacodynamic parameters for anticoagulant activity of warfarin (INRmax, AUCINR), with only a small decrease noted in INRmax of ~ 3 % with the co-administration relative to warfarin alone. INR versus time profiles across individual subjects in the study suggested some differences following co-administration with fesoterodine, although there was no definite trend with regard to the changes noted [see Drug Interactions (7.7)].
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of drug-related carcinogenicity was found in 24-month studies with oral administration to mice and rats. The highest tolerated doses in mice (females 45 to 60 mg/kg/day, males 30 to 45 mg/kg/day) correspond to 11 to 19 times (females) and 4 to 9 times (males) the estimated human AUC values reached with fesoterodine 8 mg, which is the Maximum Recommended Human Dose (MRHD). In rats, the highest tolerated dose (45 to 60 mg/kg/day) corresponds to 3 to 8 times (females) and 3 to 14 times (males) the estimated human AUC at the MRHD.
Mutagenesis
Fesoterodine was not mutagenic or genotoxic in vitro (Ames tests, chromosome aberration tests) or in vivo (mouse micronucleus test).
Impairment of Fertility
Fesoterodine had no effect on male reproductive function or fertility at doses up to 45 mg/kg/day in mice. At 45 mg/kg/day, a lower number of corpora lutea, implantation sites and viable fetuses was observed in female mice administered fesoterodine for 2-weeks prior to mating and continuing through day 7 of gestation. The maternal No-Observed-Effect Level (NOEL) and the NOEL for effects on reproduction and early embryonic development were both 15 mg/kg/day. At the NOEL, the systemic exposure, based on AUC, was 0.6 to 1.5 times higher in mice than in humans at the MRHD, whereas based on peak plasma concentrations, the exposure in mice was 5 to 9 times higher.
-
14 CLINICAL STUDIES
14.1 Adult Overactive Bladder
The efficacy of fesoterodine fumarate extended-release tablets was evaluated in two, Phase 3, randomized, double-blind, placebo-controlled, 12-week studies for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Entry criteria required that patients have symptoms of overactive bladder for ≥ 6-months duration, at least 8 micturitions per day, and at least 6 urinary urgency episodes or 3 urge incontinence episodes per 3-day diary period. Patients were randomized to a fixed dose of fesoterodine fumarate 4 or 8 mg/day or placebo. In one of these studies, 290 patients were randomized to an active control arm (an oral antimuscarinic agent). For the combined studies, a total of 554 patients received placebo, 554 patients received fesoterodine fumarate 4 mg/day, and 566 patients received fesoterodine fumarate 8 mg/day. The majority of patients were Caucasian (91%) and female (79%) with a mean age of 58 years (range 19 to 91 years).
The primary efficacy endpoints were the mean change in the number of urge urinary incontinence episodes per 24 hours and the mean change in the number of micturitions (frequency) per 24 hours. An important secondary endpoint was the mean change in the voided volume per micturition.
Results for the primary endpoints and for mean change in voided volume per micturition from the two 12-week clinical studies of fesoterodine fumarate are reported in Table 10.
Table 10: Mean Baseline and Change From Baseline to Week 12 for Urge Urinary Incontinence Episodes, Number of Micturitions, and Volume Voided per Micturition
Study 1 Study 2 Parameter Placebo
N=279Fesoterodine fumarate
4mg/day
N=265Fesoterodine fumarate
8mg/day
N=276Placebo
N=266Fesoterodine fumarate
4mg/day
N=267Fesoterodine fumarate
8mg/day
N=267Number of urge incontinence episodes per 24 hoursa Baseline 3.7 3.8 3.7 3.7 3.9 3.9 Change from baseline -1.20 -2.06 -2.27 -1.00 -1.77 -2.42 p-value vs. placebo - 0.001 <0.001 - <0.003 <0.001 Number of micturitions per 24 hours Baseline 12.0 11.6 11.9 12.2 12.9 12.0 Change from baseline -1.02 -1.74 -1.94 -1.02 -1.86 -1.94 p-value vs. placebo - <0.001 <0.001 - 0.032 <0.001 Voided volume per micturition (mL) Baseline 150 160 154 159 152 156 Change from baseline 10 27 33 8 17 33 p-value vs. placebo - <0.001 <0.001 - 0.150 <0.001 vs. = versus
a Only those patients who were urge incontinent at baseline were included for the analysis of number of urge incontinence episodes per 24 hours: In Study 1, the number of these patients was 211, 199, and 223 in the placebo, fesoterodine fumarate 4 mg/day and fesoterodine fumarate 8 mg/day groups, respectively. In Study 2, the number of these patients was 205, 228, and 218, respectively.
Figures 1 to 4: The following figures show change from baseline over time in number of micturitions and urge urinary incontinence episodes per 24 h in the two studies.
Figures 1 to 4: The following figures show change from baseline over time in number of micturitions and urge urinary incontinence episodes per 24 h in the two studies.
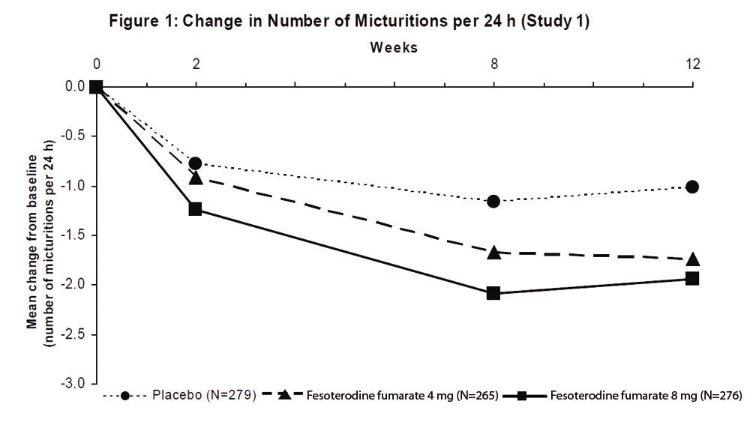
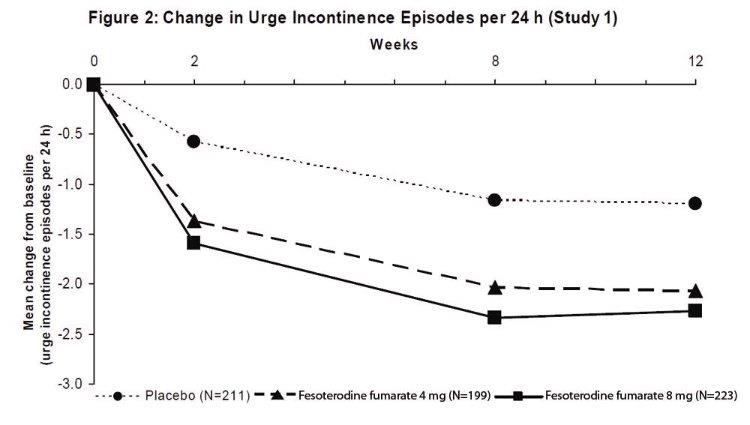
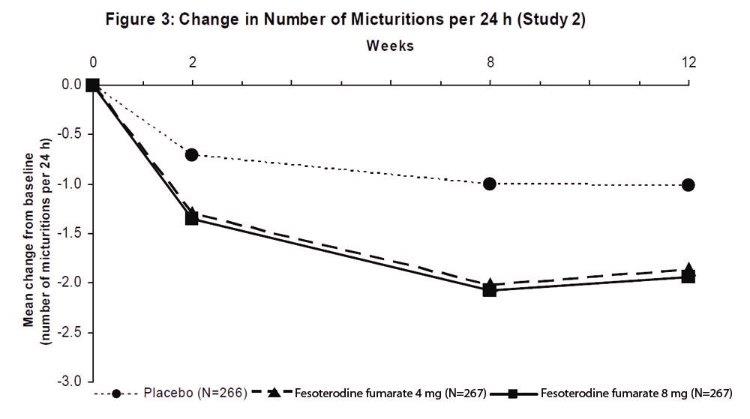
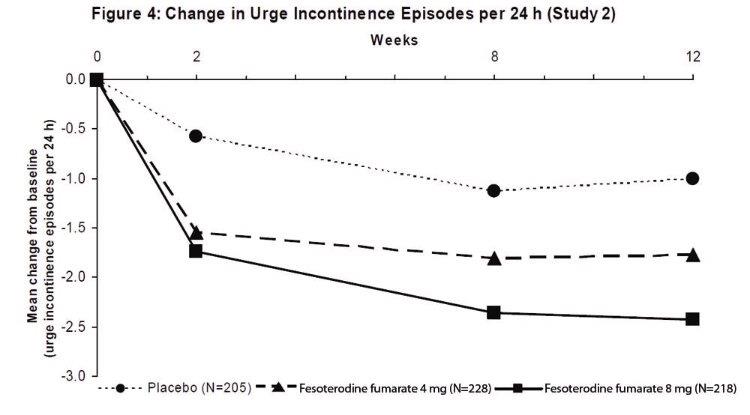
A reduction in number of urge urinary incontinence episodes per 24 hours was observed for both doses as compared to placebo as early as two weeks after starting fesoterodine fumarate therapy.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
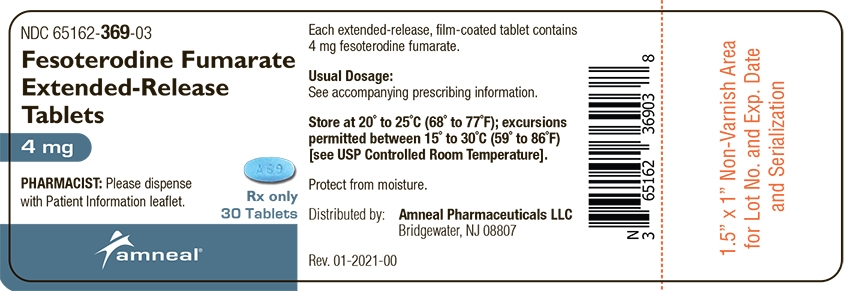
Fesoterodine fumarate extended-release tablets, 4 mg, are supplied as light blue colored, oval shaped, and film-coated; debossed with “A 69” on one side and plain on the other side.
They are available as follows:
Bottles of 30: NDC: 65162-369-03
Bottles of 90: NDC: 65162-369-09
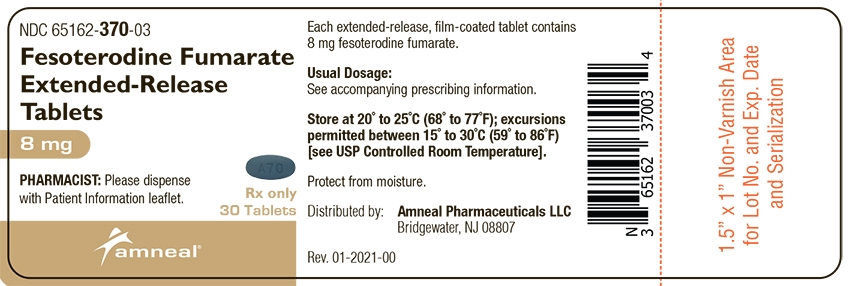
Fesoterodine fumarate extended-release tablets, 8 mg, are supplied as blue colored, oval shaped, and film-coated; debossed with “A 70” on one side and plain on the other side.
They are available as follows:
Bottles of 30: NDC: 65162-370-03
Bottles of 90: NDC: 65162-370-09
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved Patient Labeling (Patient Information).
Angioedema
Inform patients and/or their caregivers that fesoterodine may cause angioedema, which could result in life-threatening airway obstruction. Advise patients and/or their caregivers to promptly discontinue fesoterodine therapy and seek immediate medical attention if they experience edema of the lips, tongue or laryngopharynx, or difficulty breathing.
Antimuscarinic Effects
Inform patients that fesoterodine fumarate, like other antimuscarinic agents, may produce clinically significant adverse effects related to antimuscarinic pharmacological activity including constipation and urinary retention. Fesoterodine fumarate, like other antimuscarinics, may be associated with blurred vision, therefore, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effects on the patient have been determined. Heat prostration (due to decreased sweating) can occur when fesoterodine fumarate, like other antimuscarinic drugs, is used in a hot environment.
Alcohol
Patients should also be informed that alcohol may enhance the drowsiness caused by fesoterodine fumarate, like other anticholinergic agents.
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.amneal.com.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 04-2024-01
-
Patient Information
Fesoterodine (FES-oh-TER-oh-deen) Fumarate
Extended-Release Tablets, for oral use
Read the Patient Information that comes with fesoterodine fumarate extended-release tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is fesoterodine fumarate extended-release tablets?
Fesoterodine fumarate extended-release tablets are a prescription medicine used:
- in adults to treat symptoms of a condition called overactive bladder (OAB), including urge urinary incontinence (leaking or wetting accidents due to a strong need to urinate), urinary urgency (having a strong need to urinate right away), or urinary frequency (having to urinate too often).
It is not known if fesoterodine fumarate extended-release tablets are safe and effective in children younger than 6 years of age or with a body weight 55 pounds (25-kg) or less.
Who should not take fesoterodine fumarate extended-release tablets?
Do not take fesoterodine fumarate extended-release tablets if you:
- are allergic to fesoterodine fumarate extended-release tablets or any of its ingredients. See the end of this leaflet for a complete list of ingredients.
- are allergic to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules.
- are not able to empty your bladder (urinary retention).
- have delayed or slow emptying of your stomach (gastric retention).
- have an eye problem called uncontrolled narrow-angle glaucoma.
Before you take fesoterodine fumarate extended-release tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have problems emptying your bladder or you have a weak urine stream.
- have any stomach or intestinal problems, or problems with constipation.
- are receiving treatment for an eye problem called narrow-angle glaucoma.
- have a condition called Myasthenia Gravis.
- have kidney problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if fesoterodine fumarate extended-release tablets will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if fesoterodine fumarate passes into your breast milk. You should talk to your healthcare provider about the best way to feed your baby while taking fesoterodine fumarate extended-release tablets.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal products. Fesoterodine fumarate extended-release tablets may affect the way other medicines work, and other medicines may affect how fesoterodine fumarate extended-release tablets works. Especially tell your healthcare provider if you are taking antimuscarinic, antibiotics, or antifungal medicines.
Know all the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take fesoterodine fumarate extended-release tablets?
- Take fesoterodine fumarate extended-release tablets exactly as your healthcare provider tells you to take it.
- Your healthcare provider may lower your dose of fesoterodine fumarate extended-release tablets if you are an adult with severe kidney problems.
- Take fesoterodine fumarate extended-release tablets with liquid and swallow the tablet whole. Do not chew, divide, or crush the tablet.
- Take fesoterodine fumarate extended-release tablets with or without food.
- If you miss a dose of fesoterodine fumarate extended-release tablets, begin taking fesoterodine fumarate extended-release tablets again the next day. Do not take 2 doses of fesoterodine fumarate extended-release tablets in the same day.
- If you take too much fesoterodine fumarate extended-release tablets, call your healthcare provider or go to an emergency department right away.
What should I avoid while taking fesoterodine fumarate extended-release tablets?
- Fesoterodine fumarate extended-release tablets can cause blurred vision, dizziness, and drowsiness. Do not drive, operate machinery, or do other dangerous activities until you know how fesoterodine fumarate extended-release tablets affects you.
- Use caution in hot environments. Decreased sweating and severe heat illness can happen when medicines such as fesoterodine fumarate extended-release tablets are used in a hot environment.
- Drinking alcohol while taking medicines such as fesoterodine fumarate extended-release tablets may cause increased drowsiness.
What are the possible side effects of fesoterodine fumarate extended-release tablets?
Fesoterodine fumarate extended-release tablets may cause serious side effects, including:
- serious allergic reactions. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat, or tongue. If you have any of these symptoms, you should stop taking fesoterodine fumarate extended-release tablets and get emergency medical help right away.
- inability to empty bladder (urinary retention). Fesoterodine fumarate extended-release tablets may increase your chances of not being able to empty your bladder if you have bladder outlet obstruction. Tell your healthcare provider right away if you are unable to empty your bladder.
- central nervous system (CNS) effects. Talk to your healthcare provider right away if you get any of these side effects: headache, dizziness, and drowsiness.
- worsening of Myasthenia Gravis symptoms.
The most common side effects of fesoterodine fumarate extended-release tablets in adults include:
- dry mouth
- constipation
Talk to your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of fesoterodine fumarate extended-release tablets. Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store fesoterodine fumarate extended-release tablets?
- Store fesoterodine fumarate extended-release tablets at room temperature between 68° to 77°F (20° to 25°C).
- Protect the medicine from moisture by keeping the bottle closed tightly.
- Keep fesoterodine fumarate extended-release tablets and all medicines out of the reach of children.
General information about the safe and effective use of fesoterodine fumarate extended-release tablets
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fesoterodine fumarate extended-release tablets for a condition for which it was not prescribed. Do not give fesoterodine fumarate extended-release tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about fesoterodine fumarate extended-release tablets that is written for health professionals.
What are the ingredients in fesoterodine fumarate extended-release tablets?
Active ingredient: fesoterodine fumarate
Inactive ingredients: FD&C Blue No. 2, fructose granular, glyceryl behenate, hypromellose, lactose monohydrate, lecithin (soya), maize starch, polyvinyl alcohol, talc, titanium dioxide and xanthan gum.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
Trademarks are the property of their respective owners.
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.amneal.com
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807This Patient Information has been approved by the U.S. Food and Drug Administration. Rev. 04-2024-01
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FESOTERODINE FUMARATE
fesoterodine fumarate tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65162-369 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FESOTERODINE FUMARATE (UNII: EOS72165S7) (FESOTERODINE - UNII:621G617227) FESOTERODINE FUMARATE 4 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FRUCTOSE (UNII: 6YSS42VSEV) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) STARCH, CORN (UNII: O8232NY3SJ) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue (light blue) Score no score Shape OVAL Size 13mm Flavor Imprint Code A69 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65162-369-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/06/2023 2 NDC: 65162-369-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205002 01/06/2023 FESOTERODINE FUMARATE
fesoterodine fumarate tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65162-370 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FESOTERODINE FUMARATE (UNII: EOS72165S7) (FESOTERODINE - UNII:621G617227) FESOTERODINE FUMARATE 8 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FRUCTOSE (UNII: 6YSS42VSEV) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) STARCH, CORN (UNII: O8232NY3SJ) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color blue Score no score Shape OVAL Size 13mm Flavor Imprint Code A70 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65162-370-03 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/06/2023 2 NDC: 65162-370-09 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205002 01/06/2023 Labeler - Amneal Pharmaceuticals LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals of New York, LLC 123797875 analysis(65162-369, 65162-370) , label(65162-369, 65162-370) , manufacture(65162-369, 65162-370) , pack(65162-369, 65162-370)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.