Nasal Rinse Mix by ZheJiang Longmed Medical Technology Co., Ltd. Nasal Rinse Mix
Nasal Rinse Mix by
Drug Labeling and Warnings
Nasal Rinse Mix by is a Otc medication manufactured, distributed, or labeled by ZheJiang Longmed Medical Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
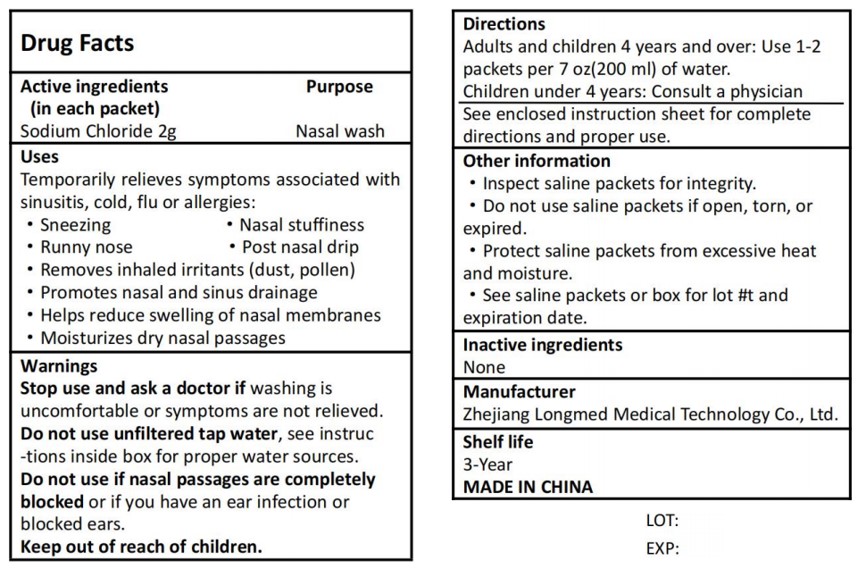
NASAL RINSE MIX- sodium chloride granule
ZheJiang Longmed Medical Technology Co., Ltd.
----------
Nasal Rinse Mix
Temporarily relieves symptoms associated withsinusitis, cold, flu or allergies:
Sneezing
Runny nose
Nasal stuffiness
Post nasal drip
Removes inhaled irritants (dust, pollen)
Promotes nasal and sinus drainage
Helps reduce swelling of nasal membranes
Moisturizes dry nasal passages
Stop use and ask a doctor if washing isuncomfortable or symptoms are not relieved
Do not use unfiltered tap water, see instruc-tions inside box for proper water sources.
Do not use if nasal passages are completelyblocked or if you have an ear infection orblocked ears.
Keep out of reach of children.
Directions
Adults and children 4 years and over: Use 1-2packets per 7 oz(200 ml) of water.Children under 4 years: Consult a physician
| NASAL RINSE MIX
sodium chloride granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ZheJiang Longmed Medical Technology Co., Ltd. (554468373) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ZheJiang Longmed Medical Technology Co., Ltd. | 554468373 | manufacture(84534-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.