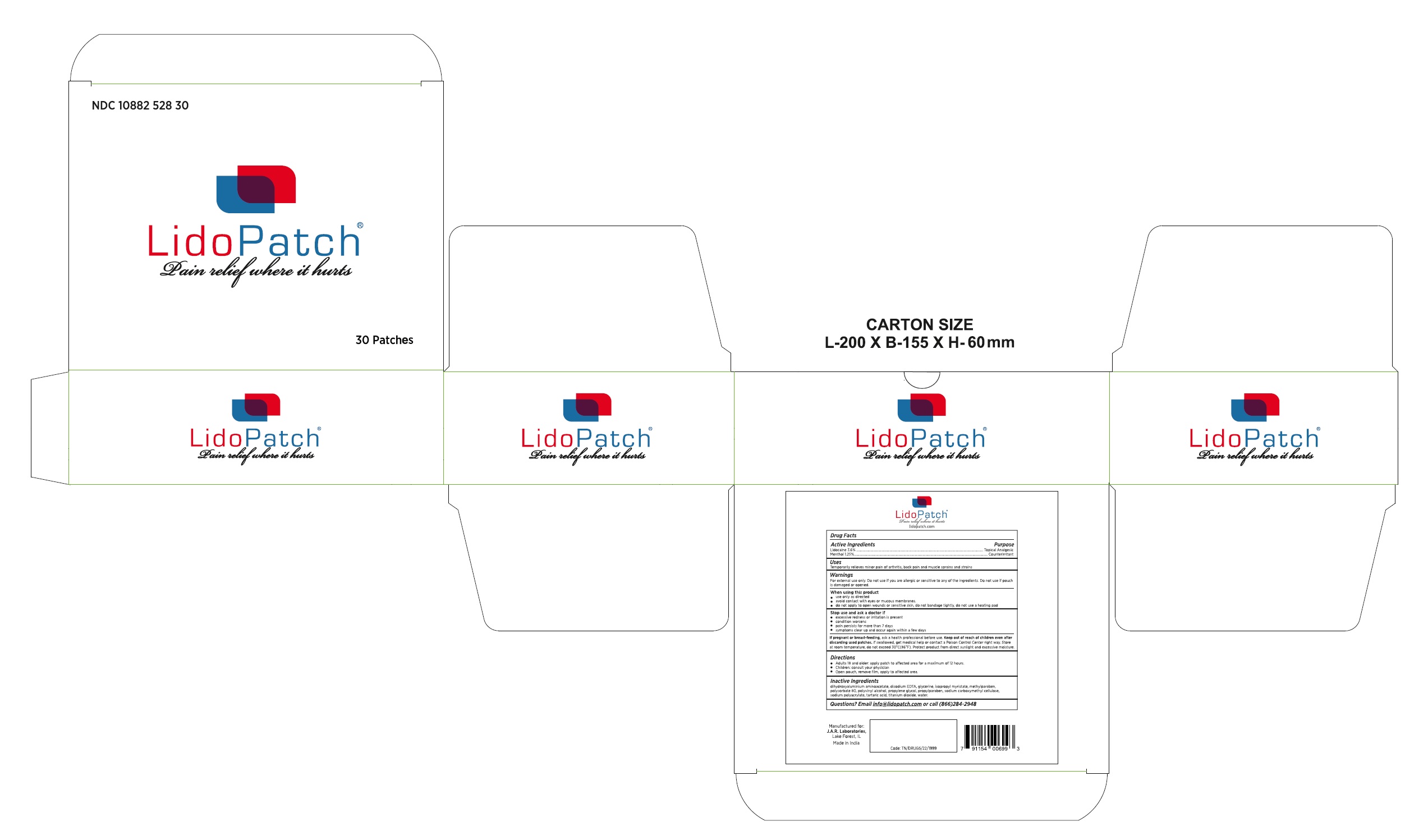
{De-Listing} LidoPatch (10882-526-30), 30 patch box, 3.6% lidocaine/1.25% menthol
LidoPatch by
Drug Labeling and Warnings
LidoPatch by is a Otc medication manufactured, distributed, or labeled by JAR Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIDOPATCH- lidocaine, menthol patch
JAR Laboratories
----------
{De-Listing} LidoPatch (10882-526-30), 30 patch box, 3.6% lidocaine/1.25% menthol
Temporarily relieves minor pain associated with: arthritis, simple backache, bursitis, tendonitis, muscle strains, sprains & bruises.
LidoPatch
contains lidocaine
PAIN RELIEF PATCH
For minor pain associated with
arthritis
back pain
muscle sprains & strains
The only over the counter patch with lidocaine
ultra thin, flexible, trim to fit
Warnings
For external use only. Do not use if you are allergic or sensitive to lidocaine or menthol. Do not use if pouch is damaged or opened.
Handling and Disposal
- hands should be washed after the handling and eye contact should be avoided
- do not store patch outside the sealed envelope
- apply immediately after removal from protected envelope
- fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get them
- kept out of the reach of children both before and after use
Stop use and ask a doctor if
- excessive redness or irritation is present
- condition worsens
- pain persists for more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or br east-feeding, ask a health professional before use.
Directions
- adults: apply patch to affected area for a maximum of 12 hours. Do not use more than 1 patch ever 24 hours.
- children: consult your physician
- remove protective film, gently apply to affected area
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
| LIDOPATCH
lidocaine, menthol patch |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - JAR Laboratories (968952239) |
Trademark Results [LidoPatch]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIDOPATCH 88345104 not registered Dead/Abandoned |
J.A.R. Laboratories 2019-03-18 |
 LIDOPATCH 85324426 4077022 Dead/Cancelled |
Ciullo, James J 2011-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.