TROPHAMINE- isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, cysteine hydrochloride, histidine, tyrosine, n-acetyl-tyrosine, alanine, arginine, proline, serine, glycine, aspartic acid, glutamic acid, and taurine solution
TrophAmine by
Drug Labeling and Warnings
TrophAmine by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
TrophAmine® (6% and 10% Amino Acid Injections) are sterile, nonpyrogenic, hypertonic solutions containing crystalline amino acids.
All amino acids designated USP are the "L"-isomer with the exception of Glycine USP, which does not have an isomer.
- * Holt LE, Snyderman SE: The amino acid requirements of infants. JAMA 1961; 175(2):124–127.
- † Rigo J, Senterre J: Is taurine essential for the neonates? Biol Neonate 1977; 32:73–76.
- ‡ Gaull G, Sturman JA, Räihä NCR: Development of mammalian sulfur metabolism: Absence of cystothionase in human fetal tissues. Pediatr Res 1972; 6:538–547.
- § Provided as acetic acid and Iysine acetate.
Each 100 mL contains: Essential Amino Acids 6% 10% Isoleucine USP 0.49 g 0.82 g Leucine USP 0.84 g 1.4 g Lysine 0.49 g 0.82 g (added as Lysine Acetate USP 0.69 g 1.2 g) Methionine USP 0.20 g 0.34 g Phenylalanine USP 0.29 g 0.48 g Threonine USP 0.25 g 0.42 g Tryptophan USP 0.12 g 0.20 g Valine USP 0.47 g 0.78 g Cysteine <0.014 g <0.016 g (as Cysteine HCl∙H2O USP <0.020 g <0.024 g) Histidine USP* 0.29 g 0.48 g Tyrosine* 0.14 g 0.24 g (added as Tyrosine USP 0.044 g 0.044 g and N-Acetyl-L-Tyrosine 0.12 g 0.24 g) Nonessential Amino Acids Alanine USP 0.32 g 0.54 g Arginine USP 0.73 g 1.2 g Proline USP 0.41 g 0.68 g Serine USP 0.23 g 0.38 g Glycine USP 0.22 g 0.36 g L-Aspartic Acid 0.19 g 0.32 g L-Glutamic Acid 0.30 g 0.50 g Taurine †,‡ 0.015 g 0.025 g Sodium Metabisulfite NF (as an antioxidant) <0.050 g <0.050 g Water for Injection USP qs qs pH adjusted with Glacial Acetic Acid USP
pH: 5.5 (5.0–6.0)Calc. Osmolarity (mOsmol/liter) 525 875 Total Amino Acids (grams/liter) 60 100 Total Nitrogen (grams/liter) 9.3 15.5 Protein Equivalent (grams/liter) 58 97 Electrolytes (mEq/liter) Sodium 5 5 §Acetate (CH3COO–) 54.4 90.7 Chloride <3 <3 -
CLINICAL PHARMACOLOGY
TrophAmine® provides a mixture of essential and nonessential amino acids as well as taurine and a soluble form of tyrosine, N-Acetyl-L-Tyrosine (NAT). This amino acid composition has been specifically formulated to provide a well tolerated nitrogen source for nutritional support and therapy for infants and young pediatric patients. When administered in conjunction with cysteine hydrochloride, TrophAmine® results in the normalization of the plasma amino acid concentrations to a profile consistent with that of a breast-fed infant.
The rationale for TrophAmine® (Amino Acid Injections) is based on the observation of inadequate levels of essential amino acids in the plasma of infants receiving total parenteral nutrition (TPN) using conventional amino acid solutions. The TrophAmine® formula was developed through the application of specific pharmacokinetic multiple regression analysis relating amino acid intake to the resulting plasma amino acid concentrations.
Clinical studies in infants and young pediatric patients who required TPN therapy showed that infusion of TrophAmine® with a cysteine hydrochloride admixture resulted in a normalization of the plasma amino acid concentrations. In addition, weight gains, nitrogen balance, and serum protein concentrations were consistent with an improving nutritional status.
When infused with hypertonic dextrose as a calorie source, supplemented with cysteine hydrochloride, electrolytes, vitamins, and minerals, TrophAmine® provides total parenteral nutrition in infants and young pediatric patients, with the exception of essential fatty acids.
It is thought that the acetate from lysine acetate and acetic acid, under the conditions of parenteral nutrition, does not impact net acid-base balance when renal and respiratory functions are normal. Clinical evidence seems to support this thinking; however, confirmatory experimental evidence is not available.
The amounts of sodium and chloride present in TrophAmine® are not of clinical significance.
The addition of cysteine hydrochloride will contribute to the chloride load.
The electrolyte content of any additives that are introduced should be carefully considered and included in total input computations.
-
INDICATIONS AND USAGE
TrophAmine® is indicated for the nutritional support of infants (including those of low birth weight) and young pediatric patients requiring TPN via either central or peripheral infusion routes. Parenteral nutrition with TrophAmine® is indicated to prevent nitrogen and weight loss or treat negative nitrogen balance in infants and young pediatric patients where (1) the alimentary tract, by the oral, gastrostomy, or jejunostomy route, cannot or should not be used, or adequate protein intake is not feasible by these routes; (2) gastrointestinal absorption of protein is impaired; or (3) protein requirements are substantially increased as with extensive burns. Dosage, route of administration, and concomitant infusion of non-protein calories are dependent on various factors, such as nutritional and metabolic status of the patient, anticipated duration of parenteral nutritional support, and vein tolerance. See WARNINGS, PRECAUTIONS, Pediatric Use, and DOSAGE AND ADMINISTRATION.
Central Venous Nutrition
Central venous infusion should be considered when amino acid solutions are to be admixed with hypertonic dextrose to promote protein synthesis in hypercatabolic or severely depleted infants, or those requiring long-term parenteral nutrition.
Peripheral Parenteral Nutrition
For moderately catabolic or depleted patients in whom the central venous route is not indicated, diluted amino acid solutions mixed with 5–10% dextrose solutions may be infused by peripheral vein, supplemented, if desired, with fat emulsion. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
-
CONTRAINDICATIONS
TrophAmine® is contraindicated in patients with untreated anuria, hepatic coma, inborn errors of amino acid metabolism, including those involving branched chain amino acid metabolism such as maple syrup urine disease and isovaleric acidemia, or hypersensitivity to one or more amino acids present in the solution.
-
WARNINGS
Safe, effective use of parenteral nutrition requires a knowledge of nutrition as well as clinical expertise in recognition and treatment of the complications which can occur. Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring of parenteral nutrition. Studies should include blood sugar, serum proteins, kidney and liver function tests, electrolytes, hemogram, carbon dioxide content, serum osmolalities, blood cultures, and blood ammonia levels.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Administration of amino acids in the presence of impaired renal function or gastrointestinal bleeding may augment an already elevated blood urea nitrogen. Patients with azotemia from any cause should not be infused with amino acids without regard to total nitrogen intake.
Administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states, or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentrations of the solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of the solutions.
Administration of amino acid solutions to a patient with hepatic insufficiency may result in plasma amino acid imbalances, hyperammonemia, prerenal azotemia, stupor and coma.
Hyperammonemia is of special significance in infants as its occurrence in the syndrome caused by genetic metabolic defects is sometimes associated, although not necessarily in a causal relationship, with mental retardation. This reaction appears to be dose related and is more likely to develop during prolonged therapy. It is essential that blood ammonia be measured frequently in infants. The mechanisms of this reaction are not clearly defined but may involve genetic defects and immature or subclinically impaired liver function.
Conservative doses of amino acids should be given, dictated by the nutritional status of the patient. Should symptoms of hyperammonemia develop, amino acid administration should be discontinued and the patient's clinical status reevaluated.
This product contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
-
PRECAUTIONS
General
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require the use of additional electrolyte supplements.
Strongly hypertonic nutrient solutions should be administered via an intravenous catheter placed in a central vein, preferably the superior vena cava.
Care should be taken to avoid circulatory overload, particularly in patients with cardiac insufficiency.
Special care must be taken when giving hypertonic dextrose to a diabetic or pre-diabetic patient. To prevent severe hyperglycemia in such patients, insulin may be required.
Administration of glucose at a rate exceeding the patient's utilization rate may lead to hyperglycemia, coma, and death.
Administration of amino acids without carbohydrates may result in the accumulation of ketone bodies in the blood. Correction of this ketonemia may be achieved by the administration of carbohydrate.
Peripheral administration of TrophAmine® (Amino Acid Injections) requires appropriate dilution and provision of adequate calories. Care should be taken to assure proper placement of the needle within the lumen of the vein. The venipuncture site should be inspected frequently for signs of infiltration. If venous thrombosis or phlebitis occurs, discontinue infusions or change infusion site and initiate appropriate treatment. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
Extraordinary electrolyte losses such as may occur during protracted nasogastric suction, vomiting, diarrhea, or gastrointestinal fistula drainage may necessitate additional electrolyte supplementation.
Metabolic acidosis can be prevented or readily controlled by adding a portion of the cations in the electrolyte mixture as acetate salts and in the case of hyperchloremic acidosis, by keeping the total chloride content of the infusate to a minimum. TrophAmine® (Amino Acid Injections) contains less than 3 mEq chloride per liter.
TrophAmine® contains no added phosphorus. Patients, especially those with hypophosphatemia, may require the addition of phosphate. To prevent hypocalcemia, calcium supplementation should always accompany phosphate administration. To assure adequate intake, serum levels should be monitored frequently.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Use only if solution is clear, the seal unbroken, and vacuum is present.
Drug product contains no more than 25 mcg/L of aluminum.
Laboratory Tests
Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring during administration.
Laboratory tests should include measurement of blood sugar, electrolyte, and serum protein concentrations; kidney and liver function tests; and evaluation of acid-base balance and fluid balance. Other laboratory tests may be suggested by the patient's condition.
Drug Interactions
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No in vitro or in vivo carcinogenesis, mutagenesis, or fertility studies have been conducted with TrophAmine®.
Pregnancy
Teratogenic Effects
Animal reproduction studies have not been conducted with TrophAmine® (Amino Acid Injections). It is also not known whether TrophAmine® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. TrophAmine® should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised with TrophAmine® if administered to a nursing woman.
Pediatric Use
As in all cases of fluid and electrolyte replacement and parenteral nutrition, careful monitoring and special caution is required in pediatric use, especially in pediatric patients with renal failure, acute sepsis, or low birth weight.
The total volume of nutritional fluid and the rate of administration in each patient will be based on individually calculated maintenance and/or replacement fluid requirements, and nutritional needs, and will vary with the child's age, body weight and renal function.
In neonates and very small infants, particularly careful monitoring will be required to maintain fluid and electrolyte balance, including monitoring of blood glucose.
See INDICATIONS AND USAGE, WARNINGS, and DOSAGE AND ADMINISTRATION.
Geriatric Use
TrophAmine® has not been studied in geriatric patients. Elderly patients are known to be more prone to fluid overload and electrolyte imbalance than younger patients. This may be related to impairment of renal function which is more frequent in an elderly population. As a result the need for careful monitoring of fluid and electrolyte therapy is greater in the elderly.
All patients, including the elderly, require an individual dose of all parenteral nutrition products to be determined by the physician on an individual case-by-case basis, which will be based on body weight, clinical condition and the results of laboratory monitoring tests. There is no specific geriatric dose.
See WARNINGS.
Special Precautions for Central Venous Nutrition
Administration by central venous catheter should be used only by those familiar with this technique and its complications.
Central venous nutrition may be associated with complications which can be prevented or minimized by careful attention to all aspects of the procedure, including solution preparation, administration, and patient monitoring. It is essential that a carefully prepared protocol, based on current medical practices, be followed, preferably by an experienced team.
Although a detailed discussion of the complications is beyond the scope of this insert, the following summary lists those based on current literature:
Technical. The placement of a central venous catheter should be regarded as a surgical procedure. One should be fully acquainted with various techniques of catheter insertion as well as recognition and treatment of complications. For details of techniques and placement sites, consult the medical literature. X-ray is the best means of verifying catheter placement. Complications known to occur from the placement of central venous catheters are pneumothorax, hemothorax, hydrothorax, artery puncture and transection, injury to the brachial plexus, malposition of the catheter, formation of arteriovenous fistula, phlebitis, thrombosis, and air and catheter embolus.
Septic. The constant risk of sepsis is present during central venous nutrition. Since contaminated solutions and infusion catheters are potential sources of infection, it is imperative that the preparation of parenteral nutrition solutions and the placement and care of catheters be accomplished under controlled aseptic conditions.
Solutions should ideally be prepared in the hospital pharmacy in a laminar flow hood. The key factor in their preparation is careful aseptic technique to avoid inadvertent touch contamination during mixing of solutions and subsequent admixtures.
Parenteral nutrition solutions should be used promptly after mixing. Any storage should be under refrigeration for as brief a time as possible. Administration time for a single bottle and set should never exceed 24 hours.
Consult the medical literature for a discussion of the management of sepsis during central venous nutrition. In brief, typical management includes replacing the solution being administered with a fresh container and set, and the remaining contents are cultured for bacterial or fungal contamination. If sepsis persists and another source of infection is not identified, the catheter is removed, the proximal tip cultured, and a new catheter reinserted when the fever has subsided. Non-specific, prophylactic antibiotic treatment is not recommended. Clinical experience indicates that the catheter is likely to be the prime source of infection as opposed to aseptically prepared and properly stored solutions.
Metabolic. The following metabolic complications have been reported: metabolic acidosis, hypophosphatemia, alkalosis, hyperglycemia and glycosuria, osmotic diuresis and dehydration, rebound hypoglycemia, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances, and hyperammonemia in pediatric patients. Frequent clinical evaluation and laboratory determinations are necessary, especially during the first few days of venous nutrition, to prevent or minimize these complications.
-
ADVERSE REACTIONS
See "WARNINGS" and "Special Precautions for Central Venous Nutrition."
Reactions reported in clinical studies as a result of infusion of the parenteral fluid were water weight gain, edema, increase in BUN, and mild acidosis.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
Local reaction at the infusion site, consisting of a warm sensation, erythema, phlebitis and thrombosis, have been reported with peripheral amino acid infusions, especially if other substances are also administered through the same site.
If electrolyte supplementation is required during peripheral infusion, it is recommended that additives be administered throughout the day in order to avoid possible venous irritation. Irritating additive medications may require injection at another site and should not be added directly to the amino acid infusate.
Symptoms may result from an excess or deficit of one or more of the ions present in the solution; therefore, frequent monitoring of electrolyte levels is essential.
Phosphorus deficiency may lead to impaired tissue oxygenation and acute hemolytic anemia. Relative to calcium, excessive phosphorus intake can precipitate hypocalcemia with cramps, tetany and muscular hyperexcitability.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The objective of nutritional management of infants and young pediatric patients is the provision of sufficient amino acid and caloric support for protein synthesis and growth.
The total daily dose of TrophAmine® (Amino Acid Injections) depends on daily protein requirements and on the patient's metabolic and clinical response. The determination of nitrogen balance and accurate daily body weights, corrected for fluid balance, are probably the best means of assessing individual protein requirements. Dosage should also be guided by the patient's fluid intake limits and glucose and nitrogen tolerances, as well as by metabolic and clinical response.
Recommendations for allowances of protein in infant nutrition have ranged from 2 to 4 grams of protein per kilogram of body weight per day (2.0 to 4.0 g/kg/day).1 The recommended dosage of TrophAmine® is 2.0 to 2.5 grams of amino acids per kilogram of body weight per day (2.0 to 2.5 g/kg/day) for infants up to 10 kilograms. For infants and young pediatric patients larger than 10 kilograms, the total dosage of amino acids should include the 20 to 25 grams/day for the first 10 kg of body weight plus 1.0 to 1.25 g/day for each kg of body weight over 10 kilograms.
Typically, TrophAmine® is admixed with B. Braun's 50% or 70% Dextrose Injection USP supplemented with electrolytes and vitamins and administered continuously over a 24 hour period.
Total daily fluid intake should be appropriate for the patient's age and size. A fluid dose of 125 mL per kilogram body weight per day is appropriate for most infants on TPN. Although nitrogen requirements may be higher in severely hypercatabolic or depleted patients, provision of additional nitrogen may not be possible due to fluid intake limits, nitrogen, or glucose intolerance.
Cysteine is considered to be an essential amino acid in infants and young pediatric patients. An admixture of cysteine hydrochloride to the TPN solution is therefore recommended. Based on clinical studies, the recommended dosage is 1.0 mmole of L-cysteine hydrochloride monohydrate per kilogram of body weight per day.
In many patients, provision of adequate calories in the form of hypertonic dextrose may require the administration of exogenous insulin to prevent hyperglycemia and glycosuria. To prevent rebound hypoglycemia, a solution containing 5% dextrose should be administered when hypertonic dextrose solutions are abruptly discontinued.
Fat emulsion coadministration should be considered when prolonged (more than 5 days) parenteral nutrition is required in order to prevent essential fatty acid deficiency (E.F.A.D.). Serum lipids should be monitored for evidence of E.F.A.D. in patients maintained on fat free TPN.
The provision of sufficient intracellular electrolytes, principally potassium, magnesium, and phosphate, is required for optimum utilization of amino acids. In addition, sufficient quantities of the major extracellular electrolytes sodium, calcium, and chloride, must be given. In patients with hyperchloremic or other metabolic acidoses, sodium and potassium may be added as the acetate salts to provide bicarbonate precursor. The electrolyte content of TrophAmine® must be considered when calculating daily electrolyte intake. Serum electrolytes, including magnesium and phosphorus, should be monitored frequently.
Appropriate vitamins, minerals and trace elements should also be provided.
Central Venous Nutrition. Hypertonic mixtures of amino acids and dextrose may be safely administered by continuous infusion through a central venous catheter with the tip located in the superior vena cava. Initial infusion rates should be slow, and gradually increased to the recommended 60–125 mL per kilogram body weight per day. If administration rate should fall behind schedule, no attempt to "catch up" to planned intake should be made. In addition to meeting protein needs, the rate of administration, particularly during the first few days of therapy, is governed by the patient's glucose tolerance. Daily intake of amino acids and dextrose should be increased gradually to the maximum required dose as indicated by frequent determinations of glucose levels in blood and urine.
Peripheral Parenteral Nutrition. For patients in whom the central venous route is not indicated and who can consume adequate calories enterally, TrophAmine® (Amino Acid Injections) may be administered by peripheral vein with or without parenteral carbohydrate calories. Such infusates can be prepared by dilution with B. Braun's Sterile Water for Injection or 5%–10% Dextrose Injection to prepare isotonic or slightly hypertonic solutions for peripheral infusion. It is essential that peripheral infusion be accompanied by adequate caloric intake. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
TrophAmine® may be admixed with solutions which contain phosphate or which have been supplemented with phosphate. The presence of calcium and magnesium ions in an additive solution should be considered when phosphate is also present, in order to avoid precipitation.
Care must be taken to avoid incompatible admixtures. Consult with pharmacist.
- 1 Suskind RM: Textbook of Pediatric Nutrition, Raven Press, New York, 1981.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
Directions for Use of B. Braun Glass Containers with Solid Stoppers
Designed for use with a vented set. Use 18 to 22 gauge needle size for admixing or withdrawing solutions from the glass bottle.
Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter; check the bottle for cracks or other damage. In checking for cracks, do not be confused by normal surface marks and seams on the bottom and sides of the bottle. These are not flaws. Look for bright reflections that have depth and penetrate into the wall of the bottle. Reject any such bottle.
-
Remove plastic cap (See Figure 1).
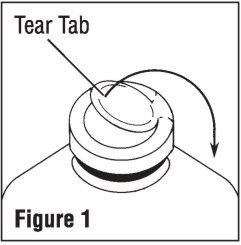
-
Swab exposed stopper surface with a suitable disinfectant.
Warning: Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. - When adding medication to the container prior to administration, swab the target area of the rubber stopper, inject medication and mix thoroughly by gentle agitation.
- Refer to Directions for Use of the set being used. Insert the set spike into the bottle through the target area of the rubber stopper. Allow the fluid to flow and remove air from the tubing before administration begins. Hang the container.
- After admixture and during administration, re-inspect the solution frequently. If any evidence of solution contamination or instability is found or if the patient exhibits any signs of fever, chills or other reactions not readily explainable, discontinue administration immediately and notify the physician.
- Spiking, additions, or transfers should be made immediately after swabbing stopper surface. Check for vacuum at first puncture of stopper. Admixture by needle or syringe should be made through the target area of the rubber stopper; contents should be drawn by vacuum into the bottle. Admixture by spiked vial should be through the target area of the rubber stopper (See Figure 2). If contents of initial addition are not drawn into the bottle, vacuum is not present and the unit should be discarded. Each addition/transfer will reduce the vacuum remaining in the bottle.
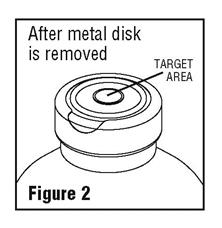
- If the first puncture of the stopper is the administration set spike, insert the spike fully into the target area of the rubber stopper and promptly invert the bottle. Verify vacuum by observing rising air bubbles. Do not use the bottle if vacuum is not present.
-
If admixture or set insertion is not performed immediately following swabbing, swab stopper surface again with a suitable disinfectant.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
TrophAmine®
(6% Amino Acid Injection)REF S9361-SS
NDC: 0264-9361-55
HK 50115500 mL
Protect from light until use.
Each 100 mL contains:
Essential Amino Acids – Isoleucine USP 0.49 g
Leucine USP 0.84 g; Lysine 0.49 g (added as Lysine Acetate USP 0.69 g)
Methionine USP 0.20 g; Phenylalanine USP 0.29 g
Threonine USP 0.25 g; Tryptophan USP 0.12 g
Valine USP 0.47 g; Cysteine <0.014 g (as Cysteine HClH20 USP <0.020 g)
Histidine USP 0.29 g; Tyrosine 0.14 g
(added as Tyrosine USP 0.044 g and N-Acetyl-L-Tyrosine 0.12 g)B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Nonessential Amino Acids -
Alanine USP 0.32 g; Arginine USP 0.73 g
Proline USP 0.41 g; Serine USP 0.23 g
Glycine USP 0.22 g; L-Aspartic Acid 0.19 g
L-Glutamic Acid 0.30 gTaurine 0.015 g; Sodium Metabisulfite NF
(antioxidant) <0.050 g; Water for Injection USP qspH adjusted with Glacial Acetic Acid USP
pH: 5.5 (5.0-6.0)
Calc. Osmolarity: 525 mOsmol/literElectrolytes (mEq/liter): Sodium 5; Chloride <3
Acetate 54.4 (see Package Insert)Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution is clear and vacuum is present.
Recommended Storage:
Room temperature (25°C). Avoid excessive heat. Protect from freezing.
See Package Insert.Rx only
TrophAmine is a registered trademark of B. Braun Medical Inc.
Y37-002-539 LD-301-2
-
PRINCIPAL DISPLAY PANEL - 500 mL Container Carton
TrophAmine®
(6% Amino Acid Injection)REF S9361-SS
NDC: 0264-9361-55
HK 50115500 mL
Protect from light until use.
Each 100 mL contains:
Essential Amino Acids –
Isoleucine USP 0.49 g; Leucine USP 0.84 g
Lysine 0.49 g (added as Lysine Acetate USP 0.69 g)
Methionine USP 0.20 g; Phenylalanine USP 0.29 g
Threonine USP 0.25 g; Tryptophan USP 0.12 g
Valine USP 0.47 g; Cysteine <0.014 g
(as Cysteine HClH20 USP <0.020 g); Histidine USP 0.29 g
Tyrosine 0.14 g (added as Tyrosine USP 0.044 g and
N-Acetyl-L-Tyrosine 0.12 g)Nonessential Amino Acids –
Alanine USP 0.32 g; Arginine USP 0.73 g
Proline USP 0.41 g; Serine USP 0.23 g
Glycine USP 0.22 g; L-Aspartic Acid 0.19 g
L-Glutamic Acid 0.30 gTaurine 0.015 g; Sodium Metabisulfite NF
(antioxidant) <0.050 g; Water for Injection USP qspH adjusted with Glacial Acetic Acid USP
pH: 5.5 (5.0-6.0)
Calculated Osmolarity: 525 mOsmol/literElectrolytes (mEq/liter):
Sodium 5 Chloride <3
Acetate 54.4 (see Package Insert)See adjacent panel for futher product information.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Sterile, nonpyrogenic. Single dose container.
For Intravenous use only.
Use only if solution is clear and vacuum is present.Recommended Storage:
Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.Rx only
TrophAmine is a registered trademark of B. Braun Medical Inc.
X12-002-491 LD-345-2

-
PRINCIPAL DISPLAY PANEL - 500 mL Container Label
TrophAmine®
(10% Amino Acid Injection)REF S9341-SS
NDC: 0264-9341-55
500 mLProtect from light until use.
Each 100 mL contains:
Essential Amino Acids - Isoleucine USP 0.82 g
Leucine USP 1.4 g; Lysine 0.82 g (added as Lysine Acetate USP 1.2 g)
Methionine USP 0.34 g; Phenylalanine USP 0.48 g
Threonine USP 0.42 g; Tryptophan USP 0.20 g
Valine USP 0.78 g; Cysteine <0.016 g (as Cysteine HClH20 USP <0.024 g)
Histidine USP 0.48 g; Tyrosine 0.24 g
(added as Tyrosine USP 0.044 g and N-Acetyl-L-Tyrosine 0.24 g)B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Nonessential Amino Acids -
Alanine USP 0.54 g; Arginine USP 1.2 g
Proline USP 0.68 g; Serine USP 0.38 g
Glycine USP 0.36 g; L-Aspartic Acid 0.32 g
L-Glutamic Acid 0.50 gTaurine 0.025 g; Sodium Metabisulfite NF
(antioxidant) <0.050 g; Water for Injection USP qspH adjusted with Glacial Acetic Acid USP
pH: 5.5 (5.0-6.0)
Calc. Osmolarity: 875 mOsmol/literElectrolytes (mEq/liter): Sodium 5; Chloride <3
Acetate 90.7 (see Package Insert)Sterile, nonpyrogenic. Single dose container.
For intravenous use only. Use only if solution is clear and
vacuum is present.Recommended Storage:
Room temperature (25°C). Avoid excessive heat. Protect from freezing.
See Package Insert.Rx only
TrophAmine is a registered trademark of B. Braun Medical Inc.
Y37-002-523 LD-299-2

-
PRINCIPAL DISPLAY PANEL - 500 mL Container Carton
TrophAmine®
(10% Amino Acid Injection)REF S9341-SS
NDC: 0264-9341-55500 mL
Protect from light until use.
Each 100 mL contains:
Essential Amino Acids –
Isoleucine USP 0.82 g; Leucine USP 1.4 g
Lysine 0.82 g (added as Lysine Acetate USP 1.2 g)
Methionine USP 0.34 g; Phenylalanine USP 0.48 g
Threonine USP 0.42 g; Typtophan USP 0.20 g
Valine USP 0.78 g; Cysteine <0.016 g
(as Cysteine HClH20 USP <0.024 g); Histidine USP 0.48 g
Tyrosine 0.24 g (added as Tyrosine USP 0.044 g and
N-Acetyl-L-Tyrosine 0.24 g)Nonessential Amino Acids –
Alanine USP 0.54 g; Arginine USP 1.2 g
Proline USP 0.68 g; Serine USP 0.38 g
Glycine USP 0.36 g; L-Aspartic Acid 0.32 g
L-Glutamic Acid 0.50 gTaurine 0.025 g; Sodium Metabisulfite NF
(antioxidant) <0.050 g; Water for Injection USP qspH adjusted with Glacial Acetic Acid USP
pH: 5.5 (5.0-6.0)
Calculated Osmolarity: 875 mOsmol/literElectrolytes (mEq/liter):
Sodium 5 Chloride <3
Acetate 90.7 (see Package Insert)See adjacent panel for further product information.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Sterile, nonpyrogenic. Single dose container.
For intravenous use only.
Use only if solution is clear and vacuum is present.Recommended Storage:
Room temperature (25°C). Avoid excessive heat.
Protect from freezing. See Package Insert.Rx only
TrophAmine is a registered trademark of B. Braun Medical Inc.
X12-002-490 LD-344-2

-
INGREDIENTS AND APPEARANCE
TROPHAMINE
isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, cysteine hydrochloride, histidine, tyrosine, n-acetyl-tyrosine, alanine, arginine, proline, serine, glycine, aspartic acid, glutamic acid, and taurine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-9361 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOLEUCINE (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) ISOLEUCINE 0.49 g in 100 mL LEUCINE (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) LEUCINE 0.84 g in 100 mL LYSINE ACETATE (UNII: TTL6G7LIWZ) (LYSINE - UNII:K3Z4F929H6) LYSINE 0.69 g in 100 mL METHIONINE (UNII: AE28F7PNPL) (METHIONINE - UNII:AE28F7PNPL) METHIONINE 0.2 g in 100 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 0.29 g in 100 mL THREONINE (UNII: 2ZD004190S) (THREONINE - UNII:2ZD004190S) THREONINE 0.25 g in 100 mL TRYPTOPHAN (UNII: 8DUH1N11BX) (TRYPTOPHAN - UNII:8DUH1N11BX) TRYPTOPHAN 0.12 g in 100 mL VALINE (UNII: HG18B9YRS7) (VALINE - UNII:HG18B9YRS7) VALINE 0.47 g in 100 mL CYSTEINE HYDROCHLORIDE (UNII: ZT934N0X4W) (CYSTEINE - UNII:K848JZ4886) CYSTEINE 0.020 g in 100 mL HISTIDINE (UNII: 4QD397987E) (HISTIDINE - UNII:4QD397987E) HISTIDINE 0.29 g in 100 mL TYROSINE (UNII: 42HK56048U) (TYROSINE - UNII:42HK56048U) TYROSINE 0.044 g in 100 mL N-ACETYLTYROSINE (UNII: DA8G610ZO5) (N-ACETYLTYROSINE - UNII:DA8G610ZO5) N-ACETYLTYROSINE 0.12 g in 100 mL ALANINE (UNII: OF5P57N2ZX) (ALANINE - UNII:OF5P57N2ZX) ALANINE 0.32 g in 100 mL ARGININE (UNII: 94ZLA3W45F) (ARGININE - UNII:94ZLA3W45F) ARGININE 0.73 g in 100 mL PROLINE (UNII: 9DLQ4CIU6V) (PROLINE - UNII:9DLQ4CIU6V) PROLINE 0.41 g in 100 mL SERINE (UNII: 452VLY9402) (SERINE - UNII:452VLY9402) SERINE 0.23 g in 100 mL GLYCINE (UNII: TE7660XO1C) (GLYCINE - UNII:TE7660XO1C) GLYCINE 0.22 g in 100 mL ASPARTIC ACID (UNII: 30KYC7MIAI) (ASPARTIC ACID - UNII:30KYC7MIAI) ASPARTIC ACID 0.19 g in 100 mL GLUTAMIC ACID (UNII: 3KX376GY7L) (GLUTAMIC ACID - UNII:3KX376GY7L) GLUTAMIC ACID 0.3 g in 100 mL TAURINE (UNII: 1EQV5MLY3D) (TAURINE - UNII:1EQV5MLY3D) TAURINE 0.015 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.05 g in 100 mL WATER (UNII: 059QF0KO0R) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-9361-55 12 in 1 CASE 07/20/1984 1 1 in 1 CARTON 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019018 07/20/1984 TROPHAMINE
isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, cysteine hydrochloride, histidine, tyrosine, n-acetyl-tyrosine, alanine, arginine, proline, serine, glycine, aspartic acid, glutamic acid, and taurine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-9341 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOLEUCINE (UNII: 04Y7590D77) (ISOLEUCINE - UNII:04Y7590D77) ISOLEUCINE 0.82 g in 100 mL LEUCINE (UNII: GMW67QNF9C) (LEUCINE - UNII:GMW67QNF9C) LEUCINE 1.4 g in 100 mL LYSINE ACETATE (UNII: TTL6G7LIWZ) (LYSINE - UNII:K3Z4F929H6) LYSINE 1.2 g in 100 mL METHIONINE (UNII: AE28F7PNPL) (METHIONINE - UNII:AE28F7PNPL) METHIONINE 0.34 g in 100 mL PHENYLALANINE (UNII: 47E5O17Y3R) (PHENYLALANINE - UNII:47E5O17Y3R) PHENYLALANINE 0.48 g in 100 mL THREONINE (UNII: 2ZD004190S) (THREONINE - UNII:2ZD004190S) THREONINE 0.42 g in 100 mL TRYPTOPHAN (UNII: 8DUH1N11BX) (TRYPTOPHAN - UNII:8DUH1N11BX) TRYPTOPHAN 0.2 g in 100 mL VALINE (UNII: HG18B9YRS7) (VALINE - UNII:HG18B9YRS7) VALINE 0.78 g in 100 mL CYSTEINE HYDROCHLORIDE (UNII: ZT934N0X4W) (CYSTEINE - UNII:K848JZ4886) CYSTEINE 0.024 g in 100 mL HISTIDINE (UNII: 4QD397987E) (HISTIDINE - UNII:4QD397987E) HISTIDINE 0.48 g in 100 mL TYROSINE (UNII: 42HK56048U) (TYROSINE - UNII:42HK56048U) TYROSINE 0.044 g in 100 mL N-ACETYLTYROSINE (UNII: DA8G610ZO5) (N-ACETYLTYROSINE - UNII:DA8G610ZO5) N-ACETYLTYROSINE 0.24 g in 100 mL ALANINE (UNII: OF5P57N2ZX) (ALANINE - UNII:OF5P57N2ZX) ALANINE 0.54 g in 100 mL ARGININE (UNII: 94ZLA3W45F) (ARGININE - UNII:94ZLA3W45F) ARGININE 1.2 g in 100 mL PROLINE (UNII: 9DLQ4CIU6V) (PROLINE - UNII:9DLQ4CIU6V) PROLINE 0.68 g in 100 mL SERINE (UNII: 452VLY9402) (SERINE - UNII:452VLY9402) SERINE 0.38 g in 100 mL GLYCINE (UNII: TE7660XO1C) (GLYCINE - UNII:TE7660XO1C) GLYCINE 0.36 g in 100 mL ASPARTIC ACID (UNII: 30KYC7MIAI) (ASPARTIC ACID - UNII:30KYC7MIAI) ASPARTIC ACID 0.32 g in 100 mL GLUTAMIC ACID (UNII: 3KX376GY7L) (GLUTAMIC ACID - UNII:3KX376GY7L) GLUTAMIC ACID 0.5 g in 100 mL TAURINE (UNII: 1EQV5MLY3D) (TAURINE - UNII:1EQV5MLY3D) TAURINE 0.025 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) 0.05 g in 100 mL WATER (UNII: 059QF0KO0R) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-9341-55 6 in 1 CASE 07/20/1984 1 1 in 1 CARTON 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019018 07/20/1984 Labeler - B. Braun Medical Inc. (002397347)
Trademark Results [TrophAmine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TROPHAMINE 78672902 not registered Dead/Abandoned |
B. Braun Medical, Inc. 2005-07-18 |
 TROPHAMINE 73425189 1281249 Live/Registered |
American Hospital Supply Corporation 1983-05-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.