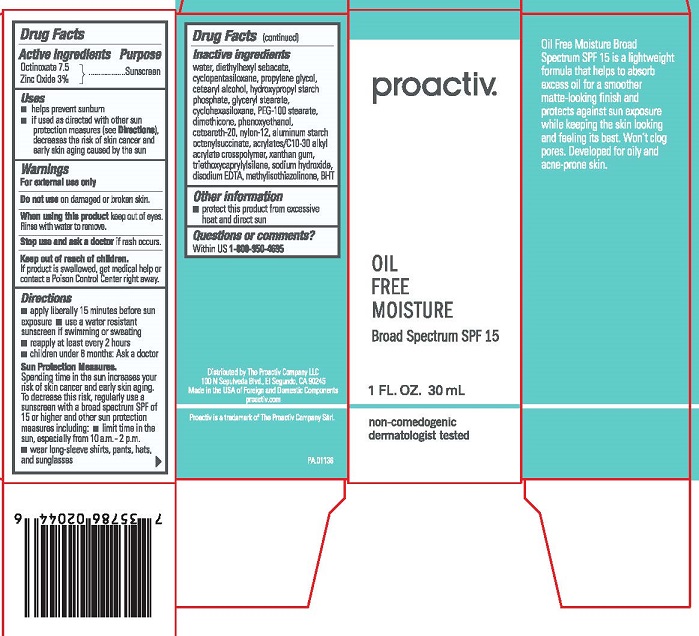
Proactiv Oil Free Moisture Broad Spectrum SPF 15
Proactiv by
Drug Labeling and Warnings
Proactiv by is a Otc medication manufactured, distributed, or labeled by THE PROACTIV COMPANY LLC, VEE PAK, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROACTIV SPF 15 MOISTURIZER- octinoxate and zinc oxide lotion
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Oil Free Moisture Broad Spectrum SPF 15
Uses
▪ helps prevent sunburn
▪ if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: ▪ limit time in the sun, especially from 10 a.m.-2 p.m. ▪ wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients
Water, Diethylhexyl Sebacate, Cyclopentasiloxane, Propylene Glycol, Cetearyl Alcohol, Hydroxypropyl Starch Phosphate, Glyceryl Stearate, Cyclohexasiloxane, PEG-100 Stearate, Dimethicone, Phenoxyethanol, Ceteareth-20, Nylon-12, Aluminum Starch Octenylsuccinate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Xanthan Gum, Triethoxycaprylylsilane, Sodium Hydroxide, Disodium EDTA, Methylisothiazolinone, BHT
Other information
▪ protect this product from excessive heat and direct sun
Questions or comments?
Within US 1-800-950-4695
Distributed by The Proactiv Company, LLC
100 N Sepulveda Blvd., El Segundo, CA 90245
Made in the USA of Foreign and Domestic Components
proactive.com
Proactiv is a trademark of The Proactiv Company Sàrl.
| PROACTIV
SPF 15 MOISTURIZER
octinoxate and zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| VEE PAK, LLC | 874763303 | manufacture(11410-603) | |
Trademark Results [Proactiv]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROACTIV 85171884 not registered Dead/Abandoned |
THE PROACTIV COMPANY SÃRL 2010-11-08 |
 PROACTIV 85023382 4143243 Dead/Cancelled |
THE PROACTIV COMPANY SÃRL 2010-04-26 |
 PROACTIV 78439806 not registered Dead/Abandoned |
AVO Multi-Amp Corporation, doing business as Megger 2004-06-23 |
 PROACTIV 78292301 not registered Dead/Abandoned |
VFS TECHNOLOGIES LIMITED 2003-08-26 |
 PROACTIV 78259207 not registered Dead/Abandoned |
ACTIV Financial Systems, Inc. 2003-06-06 |
 PROACTIV 76441664 not registered Dead/Abandoned |
E. Excel International, Inc. 2002-08-20 |
 PROACTIV 76149463 not registered Dead/Abandoned |
InfoActiv, Incorporated 2000-10-19 |
 PROACTIV 75930916 not registered Dead/Abandoned |
NATURELAND BIO PRODUCTS LTD 2000-02-29 |
 PROACTIV 75682121 2574142 Live/Registered |
THE PROACTIV COMPANY SÃRL 1999-04-13 |
 PROACTIV 75493592 not registered Dead/Abandoned |
Knightlite (U.K.) Ltd. 1998-05-29 |
 PROACTIV 74586544 2162306 Live/Registered |
THE PROACTIV COMPANY SÃRL 1994-10-17 |
 PROACTIV 74244642 not registered Dead/Abandoned |
For Women Only, Inc. 1992-02-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.