PREVAIL- flunixin meglumine injection, solution
Prevail by
Drug Labeling and Warnings
Prevail by is a Animal medication manufactured, distributed, or labeled by MWI Veterinary Supply Co, Norbrook Laboratories Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution
- Description
-
Pharmacology
Flunixin meglumine is a potent, non-narcotic, non-steroidal, analgesic agent with anti-inflammatory and anti-pyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test.
Horse: Flunixin is four times as potent on a mg per mg basis as phenylbutazone as measured by the reduction in lameness and swelling in the horse. Plasma half-life in horse serum is 1.6 hours following a single dose of 1.1 mg/kg. Measurable amounts are detectable in horse plasma at 8 hours post injection.
Cattle: Flunixin meglumine is a weak acid (pKa=5.82)1 which exhibits a high degree of plasma protein binding (approximately 99%)2. However, free (unbound) drug appears to readily partition into body tissues (VSS predictions range from 297 to 782 mL/kg2-5. Total body water is approximately equal to 570 mL/kg)6. In cattle, elimination occurs primarily through biliary excretion7. This may, at least in part, explain the presence of multiple peaks in the blood concentration/time profile following IV administration2.
In healthy cattle, total body clearance has been reported to range from 90 to 151 mL/kg/hr2-5. These studies also report a large discrepancy between the volume of distribution at steady state (VSS) and the volume of distribution associated with the terminal elimination phase (Vß). This discrepancy appears to be attributable to extended drug elimination from a deep compartment8. The terminal half-life has been shown to vary from 3.14 to 8.12 hours2-5.
Flunixin persists in inflammatory tissues9 and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentration4,9. These observations account for the counterclockwise hysteresis associated with flunixin's pharmacokinetic/pharmacodynamic relationships10. Therefore, prediction of drug concentrations based upon the estimated plasma terminal elimination half-life will likely underestimate both the duration of drug action and the concentration of drug remaining at the site of activity.
- Indications
-
Dose and Administration
Horse: The recommended dose for musculoskeletal disorders is 0.5 mg per pound (1 mL/100 lbs) of bodyweight once daily. Treatment may be given by intravenous or intramuscular injection and repeated for up to five days. Studies show onset of activity is within 2 hours. Peak response occurs between 12 and 16 hours and duration of activity is 24-36 hours.
The recommended dose for the alleviation of pain associated with equine colic is 0.5 mg per pound of bodyweight. Intravenous administration is recommended for prompt relief. Clinical studies show pain is alleviated in less than 15 minutes in many cases.
Treatment may be repeated when signs of colic recur. During clinical studies approximately 10% of the horses required one or two additional treatments. The cause of the colic should be determined and treated with concomitant therapy.
Cattle: The recommended dose for control of pyrexia associated with bovine respiratory disease and endotoxemia and control of inflammation in endotoxemia is 1.1 to 2.2 mg/kg (0.5 to 1mg/lb; 1 to 2 mL per 100 lbs) of bodyweight given by slow intravenous administration either once a day as a single dose or divided into two doses administered at 12 hour intervals for up to 3 days. The total daily dose should not exceed 2.2 mg/kg (1.0 mg/lb) of bodyweight. Avoid rapid intravenous administration of the drug.
The recommended dose for acute bovine mastitis is 2.2 mg/kg (1.0 mg/lb: 2 mL per 100 lbs) of bodyweight given once by intravenous administration.
-
Contraindications
Horse: There are no known contraindications to this drug when used as directed. Intra-arterial injection should be avoided. Horses inadvertently injected intra-arterially can show adverse reactions. Signs can be ataxia, incoordination, hyperventilation, hysteria and muscle weakness. Signs are transient and disappear without antidotal medication within a few minutes. Do not use in horses showing hypersensitivity to flunixin meglumine.
-
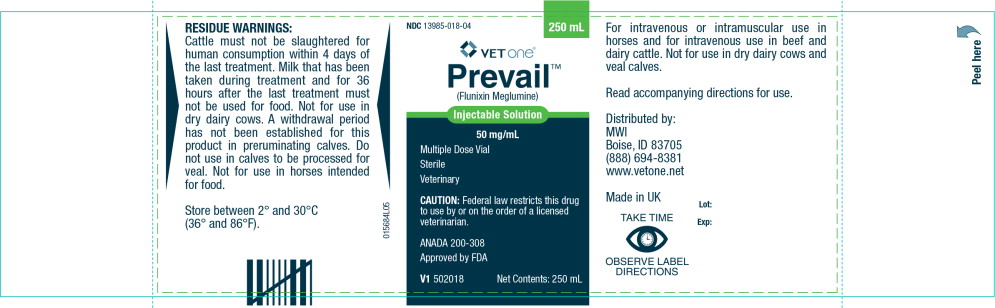
RESIDUE WARNINGS:
Cattle must not be slaughtered for human consumption within 4 days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must not be used for food. Not for use in dry dairy cows. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal. Not for use in horses intended for food.
-
Precautions
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual patient. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction.
Since many NSAIDs possess the potential to induce gastrointestinal ulceration, concomitant use of Prevail™ with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided or closely monitored.
Horse: The effects of Prevail™ on pregnancy have not been determined. Studies to determine activity of Prevail™ when administered concomitantly with other drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring adjunctive therapy.
Cattle: Do not use in bulls intended for breeding, as reproductive effects of Prevail™ in these classes of cattle have not been investigated. NSAIDs are known to have potential effects on both parturition and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. The effects of flunixin on imminent parturition have not been evaluated in a controlled study. NSAIDs are known to have the potential to delay parturition through a tocolytic effect. Do not exceed the recommended dose.
-
Safety
Horse: A 3-fold intramuscular dose of 1.5mg/lb of bodyweight daily for 10 consecutive days was safe. No changes were observed in hematology, serum chemistry, or urinalysis values. Intravenous dosages of 0.5mg/lb daily for 15 days; 1.5mg/lb daily for 10 days; and 2.5mg/lb daily for 5 days produced no changes in blood or urine parameters. No injection site irritation was observed following intramuscular injection of the 0.5mg/lb recommended dose. Some irritation was observed following a 3-fold dose administered intramuscularly.
Cattle: No flunixin-related changes (adverse reactions) were noted in cattle administered at 1X (2.2 mg/kg; 1.0 mg/lb) dose for 9 days (three times the maximum clinical duration). Minimal toxicity manifested itself at moderately elevated doses (3X and 5X) when flunixin was administered daily for 9 days, with occasional findings of blood in the feces and/or urine. Discontinue use if hematuria or fecal blood are observed.
-
Adverse Reactions
In horses, isolated reports of local reactions following intramuscular injection, particularly in the neck, have been received. These include localized swelling, sweating, induration, and stiffness. In rare instances in horses, fatal or nonfatal clostridial infections or other infections, have been reported in association with intramuscular use of Prevail™. In horses and cattle, rare instances of anaphylactic-like reactions, some of which have been fatal, have been reported, primarily following intravenous use.
- How Supplied
-
REFERENCES
- Johansson M, Anler EL. Gas chromatographic analysis of flunixin in equine urine after extractive methylation. J Chromatogr. 1988; 427:55-66
- Odensvik K, Johansson M. High-performance liquid chromatography method for determination of flunixin in bovine plasma and pharmacokinetics after single and repeated doses of the drug. Am J Vet Res. 1995; 56:489-495.
- Anderson KL, Neff-Davis CA, Davis LE, Bass VD. Pharmacokinetics of flunixin meglumine in lactating cattle after single and multiple intramuscular and intravenous administrations. Am J Vet Res. 1990; 51:1464-1467
- Odensvik K. Pharmacokinetics of flunixin and its effect on prostaglandin F2α metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacol Ther. 1995; 18:254-259.
- Hardee GE, Smith JA, Harris SJ. Pharmacokinetics of flunixin meglumine in the cow. Res Vet Sci. 1985; 39:110-112
- Ruckebusch Y, Phaneuf LP, Dunlop R. Physiology of Small and Large Animals. Chapter 2: “Body Fluid Compartments,” Philadelphia, Pa: B.C. Decker, 1991; 8-18
- Kopcha M, Ahl AS. Experimental uses of flunixin meglumine and phenylbutazone in food-producing animals. J Am Vet Med Assoc. 1989; 194:45-49
- Wagner JG. Significance of ratios of different volumes of distribution in pharmacokinetics. Biopharm & Drug Dispos. 1983; 4:263-270
- Lees P, Higgins AJ. Flunixin Inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984; 37:347-349
- Landoni MF, Cunningham FM, Lees P. Determination of pharmacokinetics and pharmacodynamics of flunixin in calves by use of pharmacokinetic/pharmacodynamic modeling. Am J Vet Res. 1995; 56:786-794.
-
RESIDUE WARNINGS:
Cattle must not be slaughtered for human consumption within 4 days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must not be used for food. Not for use in dry dairy cows. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal. Not for use in horses intended for food.
Store between 2° and 30°C (36° and 86°F).
015684L05
For intravenous or intramuscular use in horses and for intravenous use in beef and dairy cattle. Not for use in dry dairy cows and veal calves.
Read accompanying directions for use.
Distributed by:
MWI
Boise, ID 83705
(888) 694-8381
www.vetone.netMade in UK
TAKE TIME
OBSERVE LABEL DIRECTIONSLot:
Exp: - Principal Display Panel - Prevail Injectable Solution Carton Label
- Principal Display Panel - Prevail Injectable Solution Label
-
INGREDIENTS AND APPEARANCE
PREVAIL
flunixin meglumine injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13985-018 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength flunixin meglumine (UNII: 8Y3JK0JW3U) (flunixin - UNII:356IB1O400) flunixin meglumine 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium formaldehyde sulfoxylate (UNII: X4ZGP7K714) 2.5 mg in 1 mL edetate disodium (UNII: 7FLD91C86K) 0.1 mg in 1 mL diethanolamine (UNII: AZE05TDV2V) 4.0 mg in 1 mL propylene glycol (UNII: 6DC9Q167V3) 207.2 mg in 1 mL phenol (UNII: 339NCG44TV) 5.0 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-018-02 1 in 1 CARTON 1 100 mL in 1 BOTTLE, GLASS 2 NDC: 13985-018-04 1 in 1 CARTON 2 250 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200308 11/17/2003 Labeler - MWI Veterinary Supply Co (019926120) Registrant - Norbrook Laboratories Limited (214580029)
Trademark Results [Prevail]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PREVAIL 98608765 not registered Live/Pending |
Onward Watch Company, LLC 2024-06-19 |
 PREVAIL 98608749 not registered Live/Pending |
Onward Watch Company, LLC 2024-06-19 |
 PREVAIL 98590592 not registered Live/Pending |
PREVAIL GYM GEAR LLC LLC 2024-06-07 |
 PREVAIL 98495091 not registered Live/Pending |
SICURO BRANDS, LLC 2024-04-11 |
 PREVAIL 98081061 not registered Live/Pending |
Christopher Lopez 2023-07-12 |
 PREVAIL 98081061 not registered Live/Pending |
Ken Lopez 2023-07-12 |
 PREVAIL 98081061 not registered Live/Pending |
Daniel Lopez 2023-07-12 |
 PREVAIL 97888066 not registered Live/Pending |
PrevailDotLand, Inc. 2023-04-14 |
 PREVAIL 97739859 not registered Live/Pending |
Rosen's Inc. 2023-01-03 |
 PREVAIL 97546471 not registered Live/Pending |
FEED SOURCES, LLC 2022-08-12 |
 PREVAIL 97483018 not registered Live/Pending |
Columbia Insurance Company 2022-06-30 |
 PREVAIL 97365875 not registered Live/Pending |
Shook, Hardy & Bacon L.L.P. 2022-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

 Graphic Untitled
Graphic Untitled