NORTRIPTYLINE HYDROCHLORIDE capsule
Nortriptyline Hydrochloride by
Drug Labeling and Warnings
Nortriptyline Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Mayne Pharma Inc., Piramal Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of nortriptyline hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Nortriptyline hydrochloride is not approved for use in pediatric patients (see WARNINGS, Clinical Worsening and Suicide Risk; PRECAUTIONS, Information for Patients; and PRECAUTIONS, Pediatric Use).
-
DESCRIPTION
Nortriptyline hydrochloride, USP is 1-propanamine, 3-(10,11-dihydro-5H-dibenzo [a,d]cyclohepten-5-ylidene)-N-methyl-, hydrochloride. The structural formula is as follows:
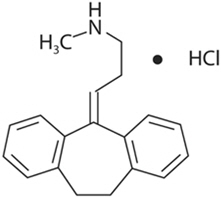
C19H21N∙HCl M.W. 299.84
Nortriptyline Hydrochloride Capsules, USP (equivalent to 10 mg, 25 mg, 50 mg and 75 mg Nortriptyline), for oral administration, contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, pregelatinized starch and sodium lauryl sulfate. The 10 mg, 25 mg, 50 mg and 75 mg capsule shells contain: gelatin, methylparaben, propylparaben, sodium lauryl sulfate and titanium dioxide. They may also contain: benzyl alcohol, butylparaben, edetate calcium disodium, silicon dioxide or sodium propionate.
The 10 mg, 25 mg and 75 mg capsule shells also contain D&C Yellow No. 10 and FD&C Blue No. 1.
-
CLINICAL PHARMACOLOGY
The mechanism of mood elevation by tricyclic antidepressants is at present unknown. Nortriptyline hydrochloride is not a monoamine oxidase inhibitor. It inhibits the activity of such diverse agents as histamine, 5-hydroxytryptamine, and acetylcholine. It increases the pressor effect of norepinephrine but blocks the pressor response of phenethylamine. Studies suggest that nortriptyline hydrochloride interferes with the transport, release, and storage of catecholamines. Operant conditioning techniques in rats and pigeons suggest that nortriptyline hydrochloride has a combination of stimulant and depressant properties.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Monoamine Oxidase Inhibitors (MAOIs)
The use of MAOIs intended to treat psychiatric disorders with nortriptyline hydrochloride or within 14 days of stopping treatment with nortriptyline hydrochloride is contraindicated because of an increased risk of serotonin syndrome. The use of nortriptyline hydrochloride within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated (see WARNINGS and DOSAGE AND ADMINISTRATION).
Starting nortriptyline hydrochloride in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome (see WARNINGS and DOSAGE AND ADMINISTRATION).
-
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
Table 1 Age Range Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated Increases Compared to Placebo <18 14 additional cases 18 to 24 5 additional cases Decreases Compared to Placebo 25 to 64 1 fewer case ≥65 6 fewer cases No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases. The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
Prescriptions for nortriptyline hydrochloride should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that nortriptyline hydrochloride is not approved for use in treating bipolar depression.
Patients with cardiovascular disease should be given nortriptyline hydrochloride only under close supervision because of the tendency of the drug to produce sinus tachycardia and to prolong the conduction time. Myocardial infarction, arrhythmia, and strokes have occurred. The antihypertensive action of guanethidine and similar agents may be blocked. Because of its anticholinergic activity, nortriptyline hydrochloride should be used with great caution in patients who have a history of urinary retention. Patients with a history of seizures should be followed closely when nortriptyline hydrochloride is administered, inasmuch as this drug is known to lower the convulsive threshold. Great care is required if nortriptyline hydrochloride is given to hyperthyroid patients or to those receiving thyroid medication, since cardiac arrhythmias may develop.
Nortriptyline hydrochloride may impair the mental and/or physical abilities required for the performance of hazardous tasks, such as operating machinery or driving a car; therefore, the patient should be warned accordingly.
Excessive consumption of alcohol in combination with nortriptyline therapy may have a potentiating effect, which may lead to the danger of increased suicidal attempts or overdosage, especially in patients with histories of emotional disturbances or suicidal ideation.
The concomitant administration of quinidine and nortriptyline may result in a significantly longer plasma half-life, higher AUC, and lower clearance of nortriptyline.
Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including nortriptyline hydrochloride, alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular changes (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
The concomitant use of nortriptyline hydrochloride with MAOIs intended to treat psychiatric disorders is contraindicated. Nortriptyline hydrochloride should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking nortriptyline hydrochloride. Nortriptyline hydrochloride should be discontinued before initiating treatment with the MAOI (see CONTRAINDICATIONS and DOSAGE AND ADMINISTRATION).
If concomitant use of nortriptyline hydrochloride with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan, and St. John's Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
Treatment with nortriptyline hydrochloride and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
Unmasking Brugada Syndrome
There have been postmarketing reports of an association between treatment with nortriptyline hydrochloride and the unmasking of Brugada syndrome. Brugada syndrome is a disorder characterized by syncope, abnormal electrocardiographic (ECG) findings, and a risk of sudden death. Nortriptyline hydrochloride should generally be avoided in patients with Brugada syndrome or those suspected of having Brugada syndrome.
Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including nortriptyline hydrochloride may trigger an angle-closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
Use in Pregnancy
Safe use of nortriptyline hydrochloride during pregnancy and lactation has not been established; therefore, when the drug is administered to pregnant patients, nursing mothers, or women of childbearing potential, the potential benefits must be weighed against the possible hazards. Animal reproduction studies have yielded inconclusive results.
-
PRECAUTIONS
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with nortriptyline hydrochloride and should counsel them in its appropriate use. A patient Medication Guide about "Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions" is available for nortriptyline hydrochloride. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking nortriptyline hydrochloride.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
The use of nortriptyline hydrochloride in schizophrenic patients may result in an exacerbation of the psychosis or may activate latent schizophrenic symptoms. If the drug is given to overactive or agitated patients, increased anxiety and agitation may occur. In manic-depressive patients, nortriptyline hydrochloride may cause symptoms of the manic phase to emerge.
Troublesome patient hostility may be aroused by the use of nortriptyline hydrochloride. Epileptiform seizures may accompany its administration, as is true of other drugs of its class.
When it is essential, the drug may be administered with electroconvulsive therapy, although the hazards may be increased. Discontinue the drug for several days, if possible, prior to elective surgery.
The possibility of a suicidal attempt by a depressed patient remains after the initiation of treatment; in this regard, it is important that the least possible quantity of drug be dispensed at any given time.
Both elevation and lowering of blood sugar levels have been reported.
Patients should be advised that taking nortriptyline hydrochloride can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Preexisting glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Drug Interactions
Administration of reserpine during therapy with a tricyclic antidepressant has been shown to produce a "stimulating" effect in some depressed patients.
Close supervision and careful adjustment of the dosage are required when nortriptyline hydrochloride is used with other anticholinergic drugs and sympathomimetic drugs.
Concurrent administration of cimetidine and tricyclic antidepressants can produce clinically significant increases in the plasma concentrations of the tricyclic antidepressant. The patient should be informed that the response to alcohol may be exaggerated.
A case of significant hypoglycemia has been reported in a type II diabetic patient maintained on chlorpropamide (250 mg/day), after the addition of nortriptyline (125 mg/day).
Drugs Metabolized by P450 2D6
The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so called "poor metabolizers"); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8 fold increase in plasma AUC of the TCA).
In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the coadministration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be coadministered with another drug known to be an inhibitor of P450 2D6.
Monoamine Oxidase Inhibitors (MAOIs)
(See CONTRAINDICATIONS, WARNINGS, and DOSAGE AND ADMINISTRATION.)
Pediatric Use
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS-Clinical Worsening and Suicide Risk). Anyone considering the use of nortriptyline hydrochloride in a child or adolescent must balance the potential risks with the clinical need.
Geriatric Use
Clinical studies of nortriptyline hydrochloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience indicates that, as with other tricyclic antidepressants, hepatic adverse events (characterized mainly by jaundice and elevated liver enzymes) are observed very rarely in geriatric patients and deaths associated with cholestatic liver damage have been reported in isolated instances. Cardiovascular function, particularly arrhythmias and fluctuations in blood pressure, should be monitored. There have also been reports of confusional states following tricyclic antidepressant administration in the elderly. Higher plasma concentrations of the active nortriptyline metabolite, 10-hydroxynortriptyline, have also been reported in elderly patients. As with other tricyclic antidepressants, dose selection for an elderly patient should usually be limited to the smallest effective total daily dose (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Note-Included in the following list are a few adverse reactions that have not been reported with this specific drug. However, the pharmacologic similarities among the tricyclic antidepressant drugs require that each of the reactions be considered when nortriptyline is administered.
Cardiovascular - Hypotension, hypertension, tachycardia, palpitation, myocardial infarction, arrhythmias, heart block, stroke.
Psychiatric - Confusional states (especially in the elderly) with hallucinations, disorientation, delusions; anxiety, restlessness, agitation; insomnia, panic, nightmares; hypomania; exacerbation of psychosis.
Neurologic - Numbness, tingling, paresthesias of extremities; incoordination, ataxia, tremors; peripheral neuropathy; extrapyramidal symptoms; seizures, alteration in EEG patterns; tinnitus.
Anticholinergic - Dry mouth and, rarely, associated sublingual adenitis; blurred vision, disturbance of accommodation, mydriasis; constipation, paralytic ileus; urinary retention, delayed micturition, dilation of the urinary tract.
Allergic - Skin rash, petechiae, urticaria, itching, photosensitization (avoid excessive exposure to sunlight); edema (general or of face and tongue), drug fever, cross-sensitivity with other tricyclic drugs.
Hematologic - Bone marrow depression, including agranulocytosis; eosinophilia; purpura; thrombocytopenia.
Gastrointestinal - Nausea and vomiting, anorexia, epigastric distress, diarrhea, peculiar taste, stomatitis, abdominal cramps, blacktongue.
Endocrine - Gynecomastia in the male, breast enlargement and galactorrhea in the female; increased or decreased libido, impotence; testicular swelling; elevation or depression of blood sugar levels; syndrome of inappropriate ADH (antidiuretic hormone) secretion.
Other - Jaundice (simulating obstructive), altered liver function; weight gain or loss; perspiration; flushing; urinary frequency, nocturia; drowsiness, dizziness, weakness, fatigue; headache; parotid swelling; alopecia.
Withdrawal Symptoms - Though these are not indicative of addiction, abrupt cessation of treatment after prolonged therapy may produce nausea, headache, and malaise.
Postmarketing Experience
The following adverse drug reaction has been reported during post-approval use of nortriptyline hydrochloride. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency.
Cardiac Disorders – Brugada syndrome
Eye Disorders – angle-closure glaucoma
To report SUSPECTED ADVERSE EVENTS, contact Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
-
OVERDOSAGE
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose, therefore, hospital monitoring is required as soon as possible.
Manifestations
Critical manifestations of overdose include: cardiac dysrhythmias, severe hypotension, shock, congestive heart failure, pulmonary edema, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity.
Other signs of overdose may include: confusion, restlessness, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the acute symptoms listed under ADVERSE REACTIONS. There have been reports of patients recovering from nortriptyline overdoses of up to 525 mg.
Management
General
Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient's airway, establish an intravenous line and initiate gastric decontamination. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. EMESIS IS CONTRAINDICATED.
Cardiovascular
A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a pC02<20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
-
DOSAGE AND ADMINISTRATION
Nortriptyline hydrochloride is not recommended for children.
Nortriptyline hydrochloride is administered orally in the form of capsules. Lower than usual dosages are recommended for elderly patients and adolescents. Lower dosages are also recommended for outpatients than for hospitalized patients who will be under close supervision. The physician should initiate dosage at a low level and increase it gradually, noting carefully the clinical response and any evidence of intolerance. Following remission, maintenance medication may be required for a longer period of time at the lowest dose that will maintain remission.
If a patient develops minor side effects, the dosage should be reduced. The drug should be discontinued promptly if adverse effects of a serious nature or allergic manifestations occur.
Usual Adult Dose - 25 mg three or four times daily; dosage should begin at a low level and be increased as required. As an alternate regimen, the total daily dosage may be given once a day. When doses above 100 mg daily are administered, plasma levels of nortriptyline should be monitored and maintained in the optimum range of 50 to 150 ng/mL. Doses above 150 mg/day are not recommended.
Elderly and Adolescent Patients - 30 to 50 mg/day, in divided doses, or the total daily dosage may be given once a day.
Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with nortriptyline hydrochloride. Conversely, at least 14 days should be allowed after stopping nortriptyline hydrochloride before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS).
Use of Nortriptyline Hydrochloride With Other MAOIs, Such as Linezolid or Methylene Blue
Do not start nortriptyline hydrochloride in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS).
In some cases, a patient already receiving nortriptyline hydrochloride therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, nortriptyline hydrochloride should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for two weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with nortriptyline hydrochloride may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS).
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with nortriptyline hydrochloride is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS).
-
HOW SUPPLIED
Nortriptyline Hydrochloride Capsules USP (equivalent to 10 mg nortriptyline) are #3, opaque deep green and opaque white capsules imprinted NORTRIPTYLINE and m 10 mg supplied in bottles of 100 and 500.
Nortriptyline Hydrochloride Capsules USP (equivalent to 25 mg nortriptyline) are #1, opaque deep green and opaque white capsules imprinted NORTRIPTYLINE and m 25 mg supplied in bottles of 100 and 500.
Nortriptyline Hydrochloride Capsules USP (equivalent to 50 mg nortriptyline) are #1, opaque white capsules imprinted NORTRIPTYLINE and m 50 mg supplied in bottles of 100 and 500.
Nortriptyline Hydrochloride Capsules USP (equivalent to 75 mg nortriptyline) are #1, opaque deep green capsules imprinted NORTRIPTYLINE and m 75 mg supplied in bottles of 100.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Nortriptyline (nor TRIP ti leen) Hydrochloride Capsules, USP
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member's antidepressant medicine. Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- 1.
Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- 2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- 3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Heart problem (Brugada Snydrome)
- If you have unexplained fainting or have a family history of sudden unexplained death before 45 years of age, you may have Brugada Syndrome and not know it.
- Talk to your healthcare provider right away if you faint or feel abnormal heartbeats.
Visual problems
- eye pain
- changes in vision
- swelling or redness in or around the eye
Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
Who should not take nortriptyline hydrochloride capsules?
Do not take nortriptyline hydrochloride capsules if you:
- take a monoamine oxidase inhibitor (MAOI). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid.
- Do not take an MAOI within 2 weeks of stopping nortriptyline hydrochloride capsules unless directed to do so by your physician.
- Do not start nortriptyline hydrochloride capsules if you stopped taking an MAOI in the last 2 weeks unless directed to do so by your physician.
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to Mayne Pharma at 1-844-825-8500 or FDA at 1-800-FDA-1088.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
- 2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- SPL UNCLASSIFIED SECTION
-
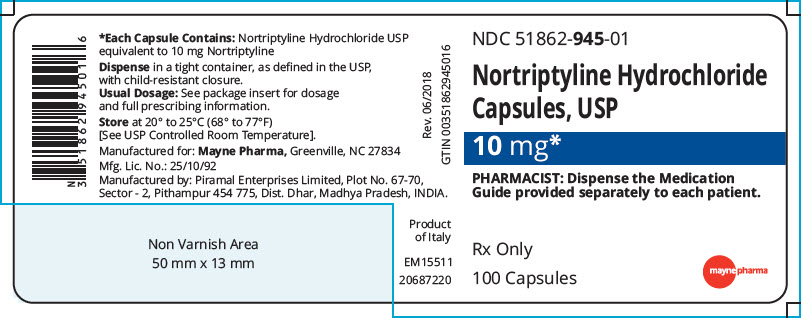
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
NDC: 51862-945-01
Nortriptyline Hydrochloride
Capsules, USP10 mg*
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.Rx Only
100 Capsules
mayne pharma
-
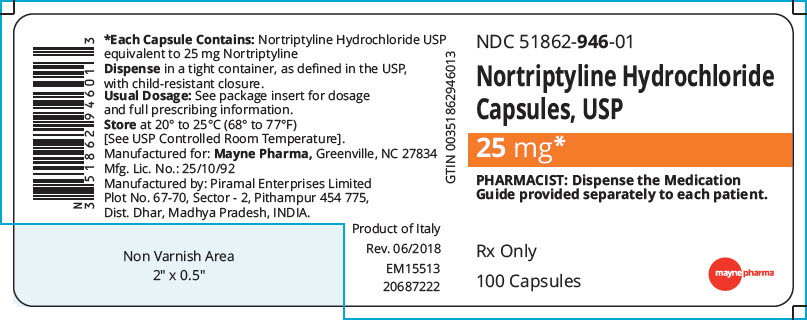
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Bottle Label
NDC: 51862-946-01
Nortriptyline Hydrochloride
Capsules, USP25 mg*
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.Rx Only
100 Capsules
mayne pharma
-
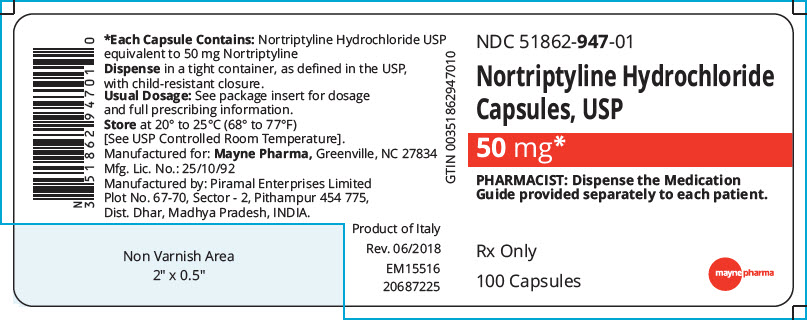
PRINCIPAL DISPLAY PANEL - 50 mg Capsule Bottle Label
NDC: 51862-947-01
Nortriptyline Hydrochloride
Capsules, USP50 mg*
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.Rx Only
100 Capsules
mayne pharma
-
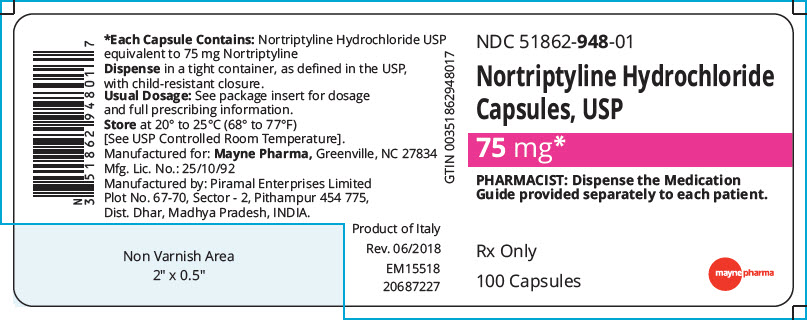
PRINCIPAL DISPLAY PANEL - 75 mg Capsule Bottle Label
NDC: 51862-948-01
Nortriptyline Hydrochloride
Capsules, USP75 mg*
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.Rx Only
100 Capsules
mayne pharma
-
INGREDIENTS AND APPEARANCE
NORTRIPTYLINE HYDROCHLORIDE
nortriptyline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-945 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nortriptyline Hydrochloride (UNII: 00FN6IH15D) (Nortriptyline - UNII:BL03SY4LXB) Nortriptyline 10 mg Inactive Ingredients Ingredient Name Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) Sodium Lauryl Sulfate (UNII: 368GB5141J) Gelatin, Unspecified (UNII: 2G86QN327L) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Titanium Dioxide (UNII: 15FIX9V2JP) Benzyl Alcohol (UNII: LKG8494WBH) Butylparaben (UNII: 3QPI1U3FV8) Edetate Calcium Disodium (UNII: 25IH6R4SGF) Sodium Propionate Hydrate (UNII: DK6Y9P42IN) D&C Yellow No. 10 (UNII: 35SW5USQ3G) FD&C Blue No. 1 (UNII: H3R47K3TBD) Starch, Corn (UNII: O8232NY3SJ) Product Characteristics Color GREEN (opaque deep green) , WHITE (opaque white) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code NORTRIPTYLINE;m;10;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-945-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 2 NDC: 51862-945-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073556 12/12/2019 NORTRIPTYLINE HYDROCHLORIDE
nortriptyline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-946 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nortriptyline Hydrochloride (UNII: 00FN6IH15D) (Nortriptyline - UNII:BL03SY4LXB) Nortriptyline 25 mg Inactive Ingredients Ingredient Name Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) Sodium Lauryl Sulfate (UNII: 368GB5141J) Gelatin, Unspecified (UNII: 2G86QN327L) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Titanium Dioxide (UNII: 15FIX9V2JP) Benzyl Alcohol (UNII: LKG8494WBH) Butylparaben (UNII: 3QPI1U3FV8) Edetate Calcium Disodium (UNII: 25IH6R4SGF) Sodium Propionate Hydrate (UNII: DK6Y9P42IN) D&C Yellow No. 10 (UNII: 35SW5USQ3G) FD&C Blue No. 1 (UNII: H3R47K3TBD) Starch, Corn (UNII: O8232NY3SJ) Product Characteristics Color GREEN (opaque deep green) , WHITE (opaque white) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code NORTRIPTYLINE;m;25;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-946-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 2 NDC: 51862-946-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073556 12/12/2019 NORTRIPTYLINE HYDROCHLORIDE
nortriptyline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-947 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nortriptyline Hydrochloride (UNII: 00FN6IH15D) (Nortriptyline - UNII:BL03SY4LXB) Nortriptyline 50 mg Inactive Ingredients Ingredient Name Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) Sodium Lauryl Sulfate (UNII: 368GB5141J) Gelatin, Unspecified (UNII: 2G86QN327L) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Titanium Dioxide (UNII: 15FIX9V2JP) Benzyl Alcohol (UNII: LKG8494WBH) Butylparaben (UNII: 3QPI1U3FV8) Edetate Calcium Disodium (UNII: 25IH6R4SGF) Sodium Propionate Hydrate (UNII: DK6Y9P42IN) Starch, Corn (UNII: O8232NY3SJ) Product Characteristics Color WHITE (opaque white) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code NORTRIPTYLINE;m;50;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-947-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 2 NDC: 51862-947-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073556 12/12/2019 NORTRIPTYLINE HYDROCHLORIDE
nortriptyline hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-948 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nortriptyline Hydrochloride (UNII: 00FN6IH15D) (Nortriptyline - UNII:BL03SY4LXB) Nortriptyline 75 mg Inactive Ingredients Ingredient Name Strength Silicon Dioxide (UNII: ETJ7Z6XBU4) Magnesium Stearate (UNII: 70097M6I30) Sodium Lauryl Sulfate (UNII: 368GB5141J) Gelatin, Unspecified (UNII: 2G86QN327L) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Titanium Dioxide (UNII: 15FIX9V2JP) Benzyl Alcohol (UNII: LKG8494WBH) Butylparaben (UNII: 3QPI1U3FV8) Edetate Calcium Disodium (UNII: 25IH6R4SGF) Sodium Propionate Hydrate (UNII: DK6Y9P42IN) D&C Yellow No. 10 (UNII: 35SW5USQ3G) FD&C Blue No. 1 (UNII: H3R47K3TBD) Starch, Corn (UNII: O8232NY3SJ) Product Characteristics Color GREEN (opaque deep green) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code NORTRIPTYLINE;m;75;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-948-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073556 12/12/2019 Labeler - Mayne Pharma Inc. (867220261) Establishment Name Address ID/FEI Business Operations Piramal Enterprises Limited 862202793 MANUFACTURE(51862-945, 51862-946, 51862-947, 51862-948) , ANALYSIS(51862-945, 51862-946, 51862-947, 51862-948) , PACK(51862-945, 51862-946, 51862-947, 51862-948) , LABEL(51862-945, 51862-946, 51862-947, 51862-948)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.