Paula's Choice Calm Redness Relief SPF 30 Mineral (Normal to Oily)
Paulas Choice Calm Redness Relief SPF 30 Mineral (Normal to Oily) by
Drug Labeling and Warnings
Paulas Choice Calm Redness Relief SPF 30 Mineral (Normal to Oily) by is a Otc medication manufactured, distributed, or labeled by Paula's Choice, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
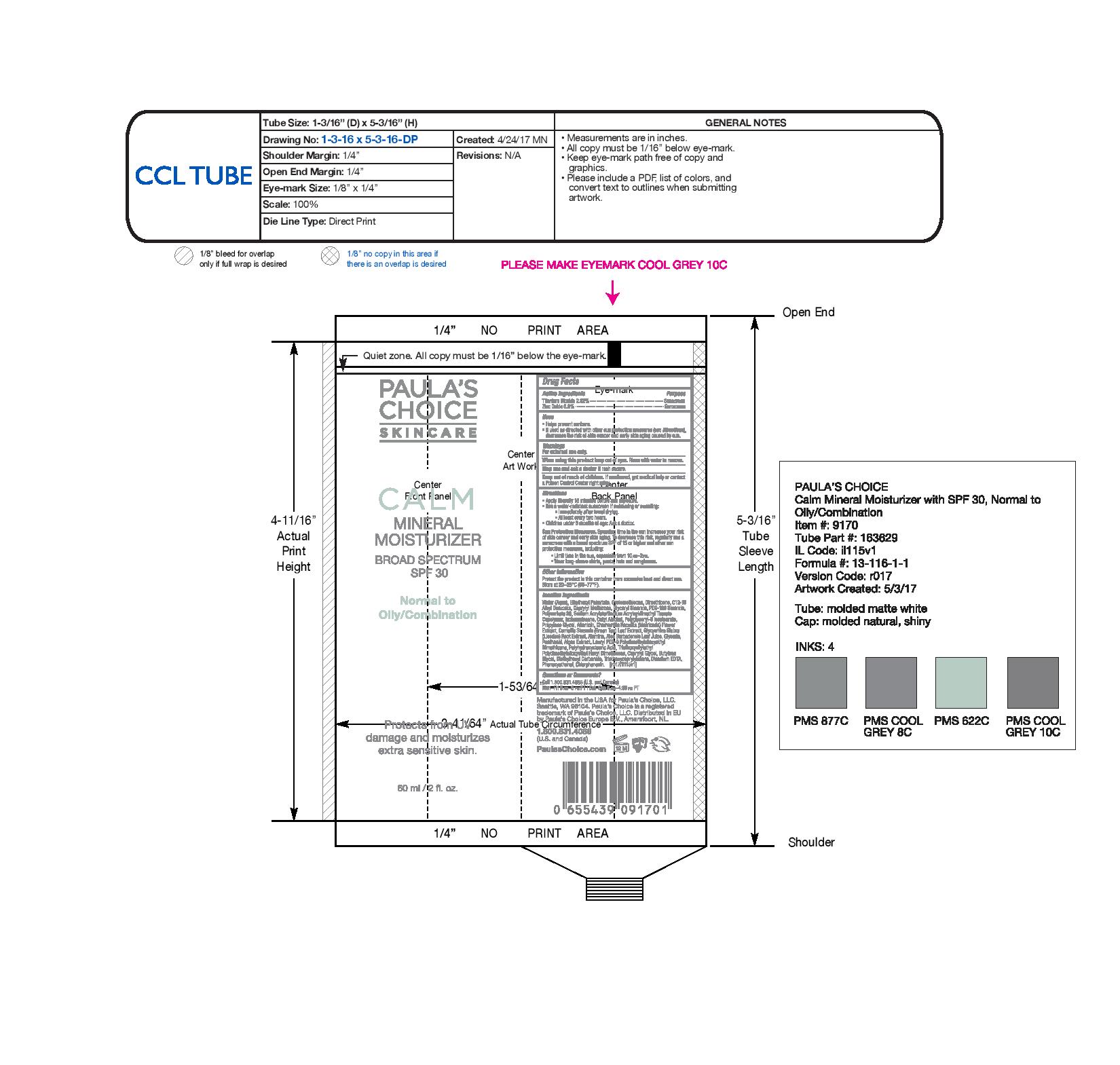
PAULAS CHOICE CALM REDNESS RELIEF SPF 30 MINERAL (NORMAL TO OILY)- zinc oxide, titanium dioxide lotion
Paula's Choice, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paula's Choice Calm Redness Relief SPF 30 Mineral (Normal to Oily)
Uses
Helps prevent sunburn. Decreases the risk of skin cancer and early skin aging caused by the sun if used as directed with other sun protection measures (see Directions)
Directions
Apply liberally 15 minutes before sun exposure. Reapply as needed or after towl drying, swimming or perspiring. Children under 6 months of age: ask a doctor. Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 25 or greater and other sun protection measures including: -limit time in the sun, espcially from 10a.m.-2p.m. -wear lnog-sleeve shirt, pants, sunglasses and a hat.
Other Information
Store at 20-25°C (68-77°F). You may report serious adverse reactions to 705 5th Avenue South, Suite 200, Seattle, WA 98104.
Water (Aqua), Ethylhexyl Palmitate, Cyclomethicone, Dimethicone, Glyceryl Stearate, PEG-100 Stearate, C12-15 Alkyl Benzoate, Polysorbate 80, Caprylyl Methicone, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Cetyl Alcohol, Propylene Glycol, Polyglyceryl-6 Isostearate, Allantoin, Chamomilla Recutita (Matricaria) Flower Extract, Camellia Sinensis (Green Tea) Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Alumina, Aloe Barbadensis (Aloe) Leaf Juice, Glycerin, Panthenol, Algae Extract, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Polyhydroxystearic Acid, Triethoxysilylethyl Poldimethylsiloxyethyl Hexyl Dimethicone, Caprylyl Glycol, Butylene Glycol, Diethylhexyl Carbonate, Triethoxycaprylylsilane, Disodium EDTA, Phenoxyethanol, Chlorphenesin.
| PAULAS CHOICE CALM REDNESS RELIEF SPF 30 MINERAL (NORMAL TO OILY)
zinc oxide, titanium dioxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice, LLC (029583981) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.