T-Relief Oral Drop
T-Relief by
Drug Labeling and Warnings
T-Relief by is a Homeopathic medication manufactured, distributed, or labeled by MediNatura Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
T-RELIEF- aconitum napellus, arnica montana, baptisia tinctoria solution/ drops
MediNatura Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
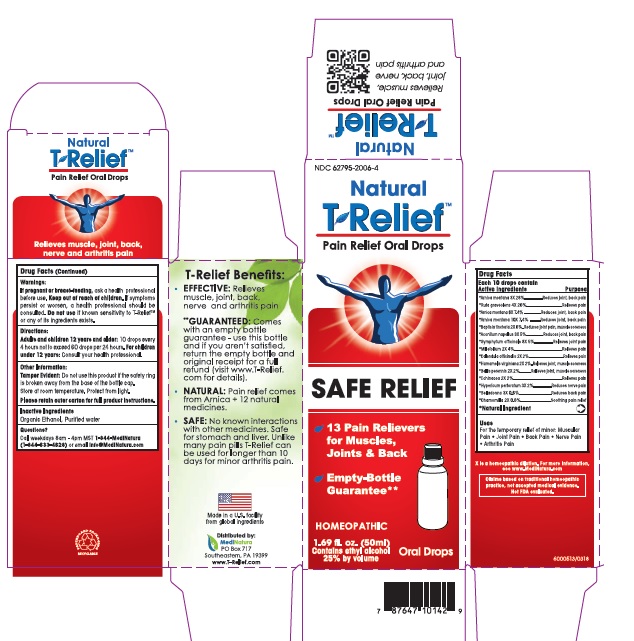
T-Relief Oral Drop
ACTIVE INGREDIENTS
*Aconitum napellus 3x, *Arnica montana 3X, 6X, 10X, Baptisia tinctoria 2X, Belladonna 3X, Bellis perennis 2X, Calendula officinalis 2X, Chamomilla 2X, Echinacea 2X, Hamamelis virginiana 2X, Hypericum perforatum 3X, Millefolium 2X, Ruta graveolens 4X, and Symphytum officinalis 8X
*Natural Ingredients
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If symptoms persist or worsen, a health professional should be consulted . Do not use if known sensitivity to T-Relief TM or any of its ingredients exists.
Directions
At first sign of symptoms: Adults and children 12 years and older: 10 drops every ½ to 1 hour until symptoms lessen, then continue with standard dosage. Do not exceed 120 drops in 24 hours. For children under 12 years: Consult your health professional.
Standard Dosage: Adults and children 12 years and older: 30 drops per day, taking 10 drops every 4 to 6 hours . For children under 12 years: Consult your health professional.
| T-RELIEF
aconitum napellus, arnica montana, baptisia tinctoria solution/ drops |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MediNatura Inc (079324099) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MediNatura Inc | 102783016 | manufacture(62795-2006) | |