ENSACOVE- ensartinib capsule
ENSACOVE by
Drug Labeling and Warnings
ENSACOVE by is a Prescription medication manufactured, distributed, or labeled by Xcovery Holdings, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENSACOVE safely and effectively. See full prescribing information for ENSACOVE.
ENSACOVETM (ensartinib) capsules, for oral use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Select patients with ALK-positive locally advanced or metastatic NSCLC for treatment with ENSACOVE. (2.1)
- Prior to initiating ENSACOVE, evaluate liver function tests and fasting blood glucose. (2.2)
- Recommended dosage: 225 mg orally once daily with or without food until disease progression or unacceptable toxicity. (2.3)
DOSAGE FORMS AND STRENGTHS
Capsules: 25 mg and 100 mg of ensartinib (3)
CONTRAINDICATIONS
Hypersensitivity reaction to ENSACOVE, FD&C Yellow No. 5 (tartrazine), or to any of its components. (4)
WARNINGS AND PRECAUTIONS
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor patients for new or worsening symptoms indicative of ILD/pneumonitis. Permanently discontinue in patients with ILD/pneumonitis. (5.1)
- Hepatotoxicity: Monitor liver function tests during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.2)
- Dermatologic Adverse Reaction: Monitor for dermatologic adverse reactions during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.3)
- Bradycardia: Monitor heart rate regularly during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.4)
- Hyperglycemia: Monitor serum glucose periodically during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.5)
- Visual Disturbances: Advise patients to report visual symptoms during treatment with ENSACOVE. Withhold ENSACOVE and obtain ophthalmologic evaluation, then reduce the dose or permanently discontinue ENSACOVE. (5.6)
- Increased Creatine Phosphokinase (CPK): Monitor CPK periodically during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.7)
- Hyperuricemia: Monitor uric acid periodically during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity. (5.8)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective contraception. (5.9)
ADVERSE REACTIONS
- Most common adverse reactions (incidence ≥20%) were rash, musculoskeletal pain, constipation, pruritus, cough, nausea, edema, vomiting, fatigue, and pyrexia. (6.1)
- Most common Grade 3-4 laboratory abnormality (incidence ≥2%) were increased uric acid, decreased lymphocytes, increased alanine aminotransferase, decreased phosphate, increased gamma glutamyl transferase, increased magnesium, increased amylase, decreased sodium, increased glucose, decreased hemoglobin, increased bilirubin, decreased potassium, and increased creatine phosphokinase. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Xcovery Holdings, Inc. at (866) 367-2268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Testing and Advice Prior to Initiating ENSACOVE
2.3 Recommended Dosage
2.4 Dosage Modification for Adverse Reactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease/Pneumonitis
5.2 Hepatotoxicity
5.3 Dermatologic Adverse Reactions
5.4 Bradycardia
5.5 Hyperglycemia
5.6 Visual Disturbances
5.7 Increased Creatine Phosphokinase
5.8 Hyperuricemia
5.9 Embryo-Fetal Toxicity
5.10 FD&C Yellow No. 5 (Tartrazine)
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on ENSACOVE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 TKI-naive ALK-Positive Locally Advanced or Metastatic NSCLC (eXALT3 Study)
16 HOW SUPPLIED
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ENSACOVE is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC)as detected by an FDA-approved test [see Dosage and Administration (2.1)] who have not previously received an ALK-inhibitor.
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of locally advanced or metastatic NSCLC with ENSACOVE based on the presence of ALK rearrangement(s) in tumor specimens [see Clinical Studies (14.1)].
Information on FDA-approved tests for the detection of ALK rearrangements in NSCLC is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Testing and Advice Prior to Initiating ENSACOVE
Prior to initiating ENSACOVE, evaluate liver function tests [see Warnings and Precautions (5.2)] and fasting blood glucose [see Warnings and Precautions (5.5)].
2.3 Recommended Dosage
The recommended dosage of ENSACOVE is 225 mg orally once daily, with or without food [see Clinical Pharmacology (12.3)], until disease progression or unacceptable toxicity.
Swallow capsules whole, do not crush or chew. Do not open or dissolve the contents of the capsule. Take ENSACOVE at the same time each day.
Missed dose
If a dose is missed, then take the missed dose as soon as possible unless the next dose is due within 12 hours. Do not take 2 doses on the same day.
Vomiting
If vomiting occurs after taking a dose, do not take an additional dose and take the next dose at its scheduled time.
2.4 Dosage Modification for Adverse Reactions
The recommended dose reductions for adverse reactions are provided in Table 1.
Table 1: Recommended Dose Reductions for Adverse Reactions Dose Reduction Recommended Dose and Schedule First 200 mg orally once daily Second 150 mg orally once daily Permanently discontinue ENSACOVE if patients are unable to tolerate 150 mg orally once daily. Once the dose has been reduced for adverse reactions, do not subsequently increase the dose of ENSACOVE.
The recommended dosage modifications for the management of adverse reactions are provided in Table 2.
Table 2: Recommended ENSACOVE Dosage Modifications for Adverse Reactions Adverse Reaction Severity* ENSACOVE Dose Modification and Management for Adverse Reactions Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.1)] Any Grade Permanently discontinue ENSACOVE. Hepatotoxicity [see Warnings and Precautions (5.2)] Grade 3 or 4 elevation (greater than 5 times ULN) of either ALT or AST with concurrent total bilirubin less than or equal to 2 times ULN - Withhold ENSACOVE until recovery to Grade ≤1 (≤3 times ULN) or to baseline.
- Resume ENSACOVE at reduced dose as per Table 1.
Grade 2 to 4 elevation (greater than 3 times ULN) of either ALT or AST with concurrent total bilirubin elevation greater than 2 times ULN in the absence of cholestasis or hemolysis Permanently discontinue ENSACOVE. Dermatologic Adverse Reactions [see Warnings and Precautions (5.3)] Grade 1 Consider topical corticosteroids. Grade 2 - Administer topical corticosteroids.
- If not improved in ≤7 days after initiation of topical corticosteroids, administer oral corticosteroids.
- If not improved in ≤7 days after initiation of oral corticosteroids, withhold ENSACOVE until recovery to Grade ≤1.
- Resume ENSACOVE at reduced dose as per Table 1.
Grade 3 - Withhold ENSACOVE. Administer topical corticosteroids.
- If not improved after 7 days of initiation of topical corticosteroids, administer oral corticosteroids.
- Resume ENSACOVE at reduced dose as per Table 1 upon improvement to Grade ≤1.
Grade 4 - Permanently discontinue ENSACOVE.
- Administer systemic corticosteroids and consider antibiotic use.
Bradycardia (HR less than 60 bpm) [see Warnings and Precautions (5.4)] Symptomatic bradycardia - Withhold ENSACOVE until recovery to asymptomatic bradycardia or to a resting heart rate of 60 bpm or above.
- If a concomitant medication known to cause bradycardia is identified and discontinued or dose-adjusted, resume ENSACOVE at same dose upon recovery to asymptomatic bradycardia or to resting heart rate of 60 bpm or above.
- If no concomitant medication known to cause bradycardia is identified, or if contributing concomitant medications are not discontinued or dose-adjusted, resume ENSACOVE at reduced dose as per Table 1 upon recovery to asymptomatic bradycardia or to resting heart rate of 60 bpm or above.
Bradycardia with life-threatening consequences, urgent intervention indicated - Permanently discontinue ENSACOVE if no contributing concomitant medication is identified.
- If contributing concomitant medication is identified and discontinued or dose- adjusted, resume ENSACOVE at reduced dose as per Table 1 upon recovery to asymptomatic bradycardia or to a resting heart rate of 60 bpm or above, with frequent monitoring as clinically indicated.
- For recurrence, permanently discontinue ENSACOVE.
Hyperglycemia [see Warnings and Precautions (5.5)] Grade 3 (greater than 250 mg/dL) despite optimal anti- hyperglycemic
therapy OR Grade 4- Withhold ENSACOVE until hyperglycemia is adequately controlled, then resume ENSACOVE at reduced dose as per Table 1.
- If adequate hyperglycemic control cannot be achieved with optimal medical management, permanently discontinue ENSACOVE.
Visual Disturbance[see Warnings and Precautions (5.6)] Grade 2 or 3 Withhold ENSACOVE until recovery to Grade 1 or baseline, then consider resuming at reduced dose as per Table 1. Grade 4 Permanently discontinue ENSACOVE. Increased Creatine Phosphokinase [see Warnings and Precautions (5.7)] CPK elevation greater than 5 times ULN - Temporarily withhold ENSACOVE until recovery to baseline or to less than or equal to 2.5 times ULN, then resume at same dose.
CPK elevation greater than 10 times ULN or second occurrence of CPK elevation of greater than 5 times ULN - Temporarily withhold ENSACOVE until recovery to baseline or to less than or equal to 2.5 times ULN, then resume at reduced dose as per Table 1.
Hyperuricemia [see Warnings and Precautions (5.8)] Symptomatic or Grade 4 - Initiate urate-lowering medication.
- Withhold ENSACOVE until improvement of signs or symptoms.
- Resume ENSACOVE at same or reduced dose.
Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 3 or 4 - Withhold ENSACOVE until recovery to Grade 1 or baseline.
- Resume ENSACOVE at reduced dose as per Table 1.
Recurrent Grade 4 Permanently discontinue ENSACOVE. ALT = alanine aminotransferase; AST = aspartate aminotransferase; bpm = beats per minute; HR = heart rate; ULN = upper limit of normal
*Graded per National Cancer Institute Common Terminology Criteria for Adverse Events. Version 4.03.
-
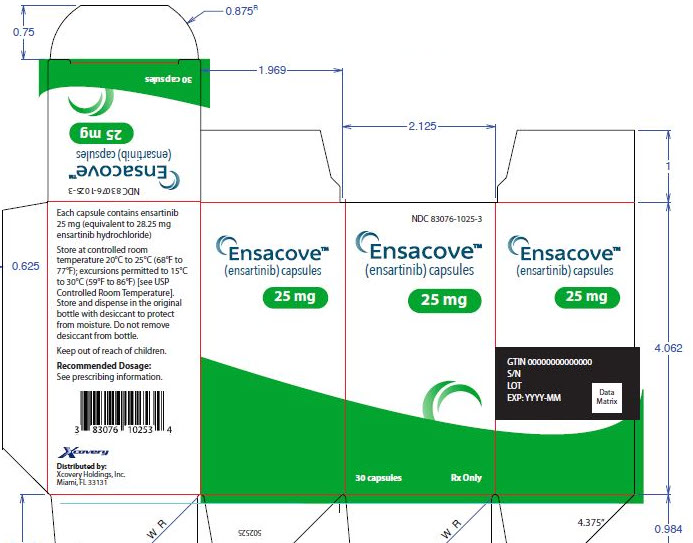
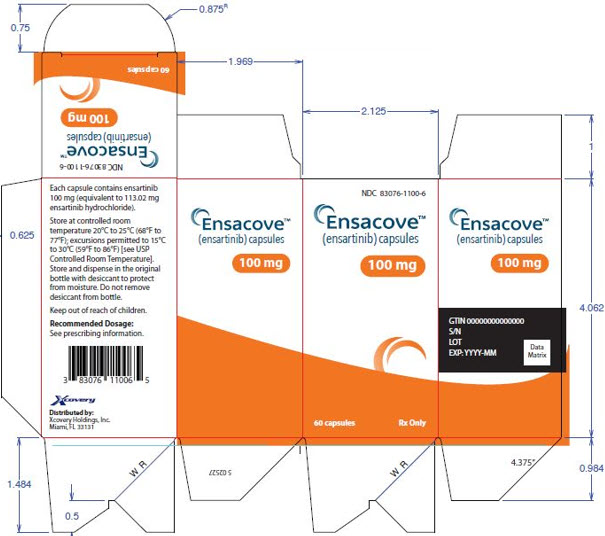
3 DOSAGE FORMS AND STRENGTHS
ENSACOVE capsules are available as:
- 25 mg: size 2 capsule, white opaque cap and body, with “X-396” on the cap and “25 mg” on the body printed in blue ink. Each capsule contains the equivalent of 25 mg of ensartinib.
- 100 mg: size 0 capsule, blue opaque cap and yellow opaque body, with “X-396” on the cap and “100 mg” on the body printed in white ink. Each capsule contains the equivalent of 100 mg of ensartinib.
-
4 CONTRAINDICATIONS
ENSACOVE is contraindicated in patients who have experienced a severe hypersensitivity reaction to ENSACOVE, FD&C Yellow No. 5 (tartrazine), or to any of its components [see Warnings and Precautions (5.10)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease/Pneumonitis
ENSACOVE can cause severe interstitial lung disease (ILD)/pneumonitis.
In the pooled safety population [see Adverse Reactions (6.1)], ILD/pneumonitis occurred in 5% of patients treated with ENSACOVE, including Grade 3 in 1.3% and Grade 4 in 0.4%. ILD/pneumonitis leading to dose interruption occurred in 0.4% and permanent discontinuation of ENSACOVE in 1.5% of patients.
Monitor patients for new or worsening symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, and fever) during treatment with ENSACOVE. Immediately withhold ENSACOVE in patients with suspected ILD/pneumonitis. Permanently discontinue ENSACOVE if ILD/pneumonitis is confirmed [see Dosage and Administration (2.4)].
5.2 Hepatotoxicity
ENSACOVE can cause hepatotoxicity including drug-induced liver injury.
In the pooled safety population [see Adverse Reactions (6.1)], 59% of patients treated with ENSACOVE had increased alanine aminotransferase (ALT), including 5% Grade 3. Increased aspartate aminotransferase (AST) occurred in 58% of patients treated with ENSACOVE, including 1.8% Grade 3. Increased bilirubin occurred in 12% of patients treated with ENSACOVE, including 2.3% Grade 3 and 0.2% Grade 4. There was one case of drug-induced liver injury in ENSACOVE-treated patients.
The median time to first onset of increased ALT or AST was 5.3 weeks (range: 0.4 to 152 weeks). The dose of ENSACOVE was interrupted in 4.6% of patients for increased ALT or AST. Increased ALT or AST leading to dose reduction occurred in 2.6% and permanent discontinuation of ENSACOVE in 1.1% of patients. The dose of ENSACOVE was interrupted in 1.3% of patients for increased bilirubin. Increased bilirubin leading to dose reduction occurred in 0.7% and permanent discontinuation of ENSACOVE in 1.3% of patients.
Monitor liver function tests including ALT, AST, and total bilirubin at baseline and every 2 weeks during the first cycle of treatment with ENSACOVE, and then monthly and as clinically indicated. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on the severity of the adverse reaction [see Dosage and Administration (2.4)].
5.3 Dermatologic Adverse Reactions
ENSACOVE can cause dermatologic adverse reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), rash, pruritus, and photosensitivity.
In the pooled safety population [see Adverse Reactions (6.1)], dermatologic adverse reactions occurred in 80% of patients receiving ENSACOVE, including Grade 3 in 14% of patients. Rash occurred in 72% of patients receiving ENSACOVE, including Grade 3 in 12% of patients. The median time to onset of rash was 9 days (range: 1 day to 17.3 months). Pruritus occurred in 32% of patients receiving ENSACOVE, with Grade 3 in 2.4%. There was one Grade 3 case (0.2%) of drug reaction with eosinophilia and systemic symptoms (DRESS).
The dose of ENSACOVE was interrupted in 12% of patients for dermatologic adverse reactions. Dermatologic adverse reactions leading to dose reduction occurred in 11% and permanent discontinuation of ENSACOVE in 1.5% of patients.
In the pooled safety population [see Adverse Reactions (6.1)], photosensitivity occurred in 0.9% of patients receiving ENSACOVE; all were Grade 1.
Monitor patients for dermatologic adverse reactions during treatment with ENSACOVE. If dermatologic adverse reactions occur, treat with antihistamine, topical or systemic steroids based on the severity. Advise patients to limit direct sun exposure while taking ENSACOVE and for at least 1 week after discontinuation. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on the severity of the adverse reaction [see Dosage and Administration (2.4)].
5.4 Bradycardia
ENSACOVE can cause symptomatic bradycardia.
In the pooled safety population [see Adverse Reactions (6.1)], bradycardia (heart rate less than 60 beats per minute) occurred in 6% of patients treated with ENSACOVE. All bradycardia events were Grade 1 or 2. Bradycardia requiring dose reduction occurred in 0.2% and led to dose interruption in 0.4% of ENSACOVE-treated patients.
Monitor heart rate regularly during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity [see Dosage and Administration (2.4)].
5.5 Hyperglycemia
ENSACOVE can cause hyperglycemia.
In the pooled safety population [see Adverse Reactions (6.1)], based on laboratory data, 44% of patients receiving ENSACOVE experienced increased blood glucose, including Grade 3 in 2.5%. The median time to onset of increased blood glucose was 5.9 weeks (0.4 weeks to 3.4 years).
Assess fasting serum glucose at baseline and monitor serum glucose periodically during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity [see Dosage and Administration (2.4)].
5.6 Visual Disturbances
ENSACOVE can cause visual disturbances including blurred vision, diplopia, photopsia, vitreous floaters, visual impairment, visual field defect, and reduced visual acuity.
In the pooled safety population [see Adverse Reactions (6.1)], 8% of patients receiving ENSACOVE experienced visual disturbance, including 0.2% Grade 3. Visual disturbances led to dose interruption in 0.4% of patients.
Obtain an ophthalmologic evaluation in patients with new or worsening visual symptoms during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity [see Dosage and Administration (2.4)].
5.7 Increased Creatine Phosphokinase
In the pooled safety population [see Adverse Reactions (6.1)], of the 203 patients with creatine phosphokinase (CPK) laboratory data available, increased CPK occurred in 43% of patients who received ENSACOVE. The incidence of Grade 3 increased CPK was 1.5% and 0.5% were Grade 4. The median time to onset of increased CPK was 123 days (range: 13 days to 22 months). Increased CPK leading to dose interruption occurred in 0.2% and dose reduction in 0.4%.
Advise patients to report any unexplained muscle pain, tenderness, or weakness. Monitor CPK levels during treatment with ENSACOVE. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity [see Dosage and Administration (2.4), Adverse Reactions (6.1)].
5.8 Hyperuricemia
ENSACOVE can cause hyperuricemia.
In the pooled safety population [see Adverse Reactions (6.1)], based on adverse reactions, 6% of patients experienced hyperuricemia, with 0.4% Grade 3 and 0.7% Grade 4. Nine patients (1.9%) required hydration and two patients (0.4%) required urate-lowering medication.
Monitor serum uric acid levels prior to initiating ENSACOVE and periodically during treatment. Initiate treatment with urate-lowering medications as clinically indicated. Withhold, reduce the dose, or permanently discontinue ENSACOVE based on severity [see Dosage and Administration (2.4)].
5.9 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, ENSACOVE can cause fetal harm when administered to a pregnant woman. In embryo-fetal developmental studies, oral administration of ensartinib to pregnant rats during the period of organogenesis caused adverse developmental outcomes, including embryo-fetal mortality, alterations to growth, and structural abnormalities. Adverse embryo-fetal findings were seen at maternal exposures approximately equivalent to the human exposure at the recommended dose of 225 mg/day based on area under the curve (AUC). Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ENSACOVE and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with ENSACOVE and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
5.10 FD&C Yellow No. 5 (Tartrazine)
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.1)]
- Hepatoxicity [see Warnings and Precautions (5.2)]
- Dermatologic Adverse Reactions [see Warnings and Precautions (5.3)]
- Bradycardia [see Warnings and Precautions (5.4)]
- Hyperglycemia [see Warnings and Precautions (5.5)]
- Visual Disturbances [see Warnings and Precautions (5.6)]
- Increased Creatine Phosphokinase [see Warnings and Precautions (5.7)]
- Hyperuricemia [see Warnings and Precautions (5.8)]
- FD&C Yellow No. 5 (Tartrazine) [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflects exposure to ENSACOVE as a single agent in 458 patients with locally advanced or metastatic ALK-positive NSCLC in the following trials: eXALT3 Study (N=143) [see Clinical Studies (14.1)], Study 101 (NCT01625234, N=98), Study BTP-28311 (NCT02959619, N=35), and Study BTP-42322 (NCT03215693, N=182). Patients received ENSACOVE 225 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. Among 458 patients who received ENSACOVE, 63% were exposed for 6 months or longer and 47% were exposed for greater than one year. In this pooled safety population, the most common adverse reactions (≥20%) were rash, musculoskeletal pain, constipation, pruritus, cough, nausea, edema, vomiting, fatigue, and pyrexia. The most frequent Grade 3 or 4 laboratory abnormalities (≥2%) were increased uric acid, decreased lymphocytes, increased alanine aminotransferase, decreased phosphate, increased gamma glutamyl transferase, increased magnesium, increased amylase, decreased sodium, increased glucose, decreased hemoglobin, increased bilirubin, decreased potassium, and increased creatine phosphokinase.
TKI-naive ALK-Positive Locally Advanced or Metastatic NSCLC
The safety of ENSACOVE was evaluated in the eXALT3 study [see Clinical Studies (14.1)]. Patients received ENSACOVE 225 mg orally once daily, with or without food, until disease progression or unacceptable toxicity. Among patients who received ENSACOVE, 78% were exposed for 6 months or longer and 66% were exposed for greater than one year.
The median age of patients who received ENSACOVE was 54 years (range: 25-86); 50% male; 54% Asian, 43% White; 0.7% Black or African American; and 11% Hispanic or Latino.
Serious adverse reactions occurred in 23% of patients treated with ENSACOVE. Serious adverse reactions that occurred in ≥1% were pneumonia (4.9%), hemorrhage (2.1%), rash (2.1%) and cellulitis (1.4%). One fatal adverse reaction (0.7%) occurred due to bronchopneumonia.
Permanent discontinuation of ENSACOVE due to an adverse reaction occurred in 12% of patients. Adverse reactions which resulted in permanent discontinuation of ENSACOVE (≥1%) included increased blood bilirubin (1.4%), increased conjugated bilirubin (1.4%), increased ALT (2.1%), increased AST (2.1%), and pneumonitis/ILD (2.1%).
Dose interruptions of ENSACOVE due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dose interruptions (≥2%) included rash (13%), increased ALT (6%), edema (2.8%), pruritus (2.8%), pyrexia (2.8%), pneumonia (3.5%), increased AST (2.1%), hemorrhage (2.1%), and decreased appetite (2.1%).
Dose reductions of ENSACOVE due to an adverse reaction occurred in 24% of patients. Adverse reactions which required dose reductions (≥2%) included rash (11%), increased ALT (4.2%), pruritus (2.8%), and edema (2.1%).
Tables 3 and 4 summarize the most frequent adverse reactions and laboratory abnormalities, respectively.
Table 3: Adverse Reactions ( ≥10%) in Patients with ALK-Positive Locally Advanced or Metastatic NSCLC Who Received ENSACOVE in eXALT3 Study ENSACOVE
N = 143Crizotinib
N = 146Adverse Reaction All Grades
%Grade 3 or 4
%All Grades
%Grade 3 or 4
%Skin and Subcutaneous Tissue Disorders Rasha 66 12 10 0 Pruritusb 30 2.1 4.1 0 Alopecia 11 0 4.8 0 Dry Skin 10 0.7 0.7 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal Painc 36 1.4 20 0 Respiratory, Thoracic and Mediastinal Disorders Coughd 31 0.7 16 0 Gastrointestinal Disorders Constipation 31 0 26 0 Nausea 28 1.4 30 2.1 Vomitinge 16 0.7 32 0 General Disorders and Administration Site Conditions Edemaf 27 2.1 28 2.1 Pyrexiag 22 0.7 10 0.7 Fatigueh 21 0.7 14 1.4 Metabolism and Nutrition Disorders Decreased appetite 15 0 12 1.4 Infection and Infestation Respiratory Tract Infection 13 0.7 10 0 Nervous System Disorders Dizzinessi 12 0.7 14 0.7 Dysgeusia 10 0 11 0 Vascular Disorders Hemorrhagej 10 1.4 4.8 0 Adverse reactions were graded using NCI CTCAE version 4.03. a Includes dermatitis, dermatitis acneiform, dermatitis bullous, drug eruption, eczema, exfoliative rash, palmar-plantar erythrodysaesthesia, rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash morbilliform, rash papular, rash pruritic, rash pustular, skin exfoliation, and vulvovaginal rash
b Includes ear pruritus, eye pruritus, eyelids pruritus, lip pruritus, pruritus, and pruritus generalized
c Includes arthritis, spinal pain, myalgia, musculoskeletal pain, back pain, pain in extremity, neck pain, arthralgia, non-cardiac chest pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort
d Includes cough, productive cough, upper-airway cough syndrome
e Includes vomiting and retching
f Includes eyelid edema, face edema, generalized edema, localized edema, edema, edema peripheral, gravitational edema, skin edema, eye edema, and periorbital edema
g Includes pyrexia and hyperthermia
h Includes fatigue and asthenia
i Includes dizziness, vertigo, postural dizziness
j Includes hemoptysis, intracranial hemorrhage, gastrointestinal hemorrhage, hematuria, upper gastrointestinal hemorrhage, vaginal hemorrhage, gingival bleeding, vitreous hemorrhage, epistaxis, rectal hemorrhage, anal hemorrhage
Table 4: Select Laboratory Abnormalities (≥20%) That Worsened from Baseline in Patients with ALK-Positive Locally Advanced or Metastatic NSCLC Who Received ENSACOVE in eXALT3 Study ENSACOVE
N = 143Crizotinib
N = 146Lab Abnormality All Grades
%Grade 3 or 4
%All Grades
%Grade 3 or 4
%Chemistry Alanine aminotransferase increased
73 5 74 8 Alkaline phosphatase increased 64 2.2 50 0.7 Aspartate aminotransferase increased 64 1.4 62 3.5 Glucose increased 49 5 35 0.7 Albumin decreased 46 0.7 56 1.4 Phosphate decreased 39 7 42 4.9 Urate increased 39 39 27 27 Creatinine increased 37 0 27 0 Calcium decreased 36 1.4 64 4.9 Sodium decreased 27 4.3 27 4.2 Hematology Lymphocytes decreased 57 7 47 5 Hemoglobin decreased 43 0.7 31 1.4 Adverse reactions were graded using NCI CTCAE version 4.03.
ALT = Alanine aminotransferase; AST = Aspartate aminotransferase
Clinically relevant adverse reactions in <10% of patients who received ENSACOVE included interstitial lung disease, photosensitivity, increased creatinine phosphokinase, bradycardia, and visual disturbances.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on ENSACOVE
Table 5 describes drug interactions where concomitant use of another drug affects ENSACOVE.
Table 5: Effect of Other Drugs on ENSACOVE Strong or Moderate CYP3A Inhibitors Prevention or Management Avoid concomitant use of strong or moderate CYP3A inhibitors with ENSACOVE. Mechanism and Clinical Effect(s) This recommendation is based upon a mechanistic understanding of ensartinib pharmacokinetics and it being a CYP3A4 substrate in vitro [see Clinical Pharmacology (12.3)]. Concomitant use with strong or moderate CYP3A inhibitors may increase ensartinib exposure; however, this has not been studied clinically. Strong or Moderate CYP3A Inducers Prevention or Management Avoid concomitant use of strong or moderate CYP3A inducers with ENSACOVE. Mechanism and Clinical Effect(s) This recommendation is based upon a mechanistic understanding of ensartinib pharmacokinetics and it being a CYP3A4 substrate in vitro [see Clinical Pharmacology (12.3)]. Concomitant use with strong or moderate CYP3A inducers may decrease ensartinib exposure; however, this has not been studied clinically. P-gp Inhibitors Prevention or Management Avoid concomitant use of P-gp inhibitors with ENSACOVE. Mechanism and Clinical Effect(s) This recommendation is based upon a mechanistic understanding of ensartinib pharmacokinetics and it being a P-gp substrate in vitro [see Clinical Pharmacology (12.3)]. Concomitant use with P-gp inhibitors may increase ensartinib exposure; however, this has not been studied clinically.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], ENSACOVE can cause fetal harm when administered to a pregnant woman. There are no available data on the use of ENSACOVE in pregnant women to inform a drug-associated risk. Oral administration of ensartinib to pregnant rats during the period of organogenesis caused adverse developmental outcomes, including embryo-fetal mortality, alterations to growth, and structural abnormalities. Adverse embryo-fetal findings were seen at maternal exposures approximately equivalent to the human exposure at the recommended dose of 225 mg/day based on AUC (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In an embryo-fetal development study, pregnant rats received 20, 40, or 80 mg/kg/day of ensartinib during the period of organogenesis (gestation day 6 to 17). Ensartinib doses ≥40 mg/kg/day (approximately equivalent to the human exposure at the recommended dose of 225 mg/day based on AUC) resulted in an increase in the incidence of fetal malformations (aortic dislocation, ventricular septal defect, separated vertebrae) and dose-dependent delayed skeletal development, including decreased or delayed ossification of the vertebrae. Ensartinib at 80 mg/kg (approximately 6.4 times the human exposure at the recommended dose of 225 mg/day based on AUC) resulted in maternal toxicity (reduced body weight and food consumption), decreased fetal body weight, increased pre- and post-implantation loss, decreases in the number of live fetuses, and visceral variations (missing renal papillae, pyelectasis).
8.2 Lactation
Risk Summary
There are no data on the presence of ensartinib or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with ENSACOVE and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
ENSACOVE can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating ENSACOVE.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with ENSACOVE and for at 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENSACOVE and for 1 week after the last dose.
8.4 Pediatric Use
The safety and effectiveness of ENSACOVE in pediatric patients have not been established.
8.5 Geriatric Use
Of the 458 patients enrolled in clinical studies and received ENSACOVE 225 mg once daily, 16% of the participants were aged 65 years or older. Clinical studies of ENSACOVE did not include sufficient numbers of patients ages 65 and over to determine whether they respond differently from younger patients. Exploratory analysis suggests a higher incidence of serious adverse events (43% vs 27%), more frequent adverse events leading to treatment discontinuations (18% vs 10%) and dose modifications (34% vs 16%) in patients 65 years or older as compared to those younger than 65 years.
8.6 Hepatic Impairment
Ensartinib is primarily metabolized by the liver and patients with hepatic impairment may have increased exposures [see Clinical Pharmacology (12.3)]. Avoid use of ENSACOVE for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST) since it has not been studied in this population. Monitor patients with moderate hepatic impairment (total bilirubin >1.5 to ≤ 3 ULN and any AST) for increased adverse reactions and adjust ENSACOVE dosage as clinically indicated [see Dosage and Administration (2.4), Warnings and Precautions (5.2)]. No dosage modification is recommended for patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and AST > ULN or total bilirubin 1 to 1.5 x ULN and any AST).
-
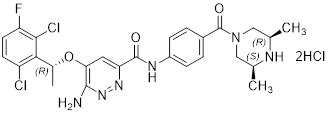
11 DESCRIPTION
ENSACOVE capsules contain ensartinib, a kinase inhibitor, present as ensartinib hydrochloride with the chemical name 6-amino-5-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-N-{4-[(3R,5S)- 3,5-dimethylpiperazine-1-carbonyl]phenyl}pyridazine-3-carboxamide, dihydrochloride. The molecular formula is C26H27Cl2FN6O3·2HCl and its molecular weight is 634.4 g/mol with the following structure:
ENSACOVE capsules are intended for oral administration and are available in two dosage strengths: 25 mg ensartinib (equivalent to 28.25 mg ensartinib hydrochloride) and 100 mg ensartinib (equivalent to 113.02 mg ensartinib hydrochloride).
The inactive ingredients of ENSACOVE capsules are microcrystalline cellulose and stearic acid. The inactive ingredients of the 25 mg empty capsule shells are hypromellose and titanium dioxide. The inactive ingredients of the 100 mg empty capsules shells are black iron oxide, FD&C Blue No. 1, FD&C Yellow No. 5, hypromellose, red iron oxide, and titanium dioxide.
The imprinting ink for the 25 mg capsules contains butyl alcohol, dehydrated alcohol, FD&C Blue No. 2, isopropyl alcohol, propylene glycol, shellac, and strong ammonia solution. The imprinting ink for the 100 mg capsules contains butyl alcohol, dehydrated alcohol, isopropyl alcohol, povidone, propylene glycol, shellac, sodium hydroxide, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ensartinib is a kinase inhibitor of anaplastic lymphoma kinase (ALK) and inhibits other kinases including MET and ROS1. In vitro, ensartinib inhibited phosphorylation of ALK and its downstream signaling proteins AKT, ERK, and S6, thereby blocking ALK-mediated signaling pathways and inhibiting proliferation in cell lines harboring ALK fusions and mutations. In vivo, ensartinib showed anti-tumor activity in a mouse xenograft model of human NSCLC harboring an ALK fusion.
12.2 Pharmacodynamics
Exposure-response relationship
Ensartinib exposure-response relationships and the time course of the pharmacodynamic response have not been fully characterized.
Cardiac Electrophysiology
At the approved recommended dosage, a mean increase in the QTc interval > 20 ms was not observed.
12.3 Pharmacokinetics
Ensartinib mean (coefficient of variation [CV%]) maximum concentration (Cmax) is 292 ng/mL (60%), and the area under the concentration-time curve (AUC0–24h) is 4,920 ng·h /ml (62%) at the approved recommended dosage. Ensartinib steady state is reached within 15 days with a mean accumulation ratio of 2.7.
Absorption
Ensartinib median (minimum, maximum) time to reach Cmax (Tmax) at steady state is 3 hours (2, 8 hours).
Effect of Food
No clinically significant differences in ensartinib pharmacokinetics were observed following administration of ENSACOVE with a high-fat meal (total 800-1000 calories, > 50% fat) compared to fasted conditions.
Distribution
Ensartinib mean (CV%) apparent volume of distribution is 1,720 L (42%). Ensartinib is 91.6% bound to human plasma protein.
Elimination
Ensartinib mean (standard deviation [SD]) steady-state half-life (t1/2) is 30 (20) hours.
Metabolism
Ensartinib is predominantly metabolized by CYP3A.
Excretion
Following a single oral 200 mg dose of radiolabeled ensartinib, 91% of the radioactivity was recovered in feces (38% as unchanged) and 10% in urine (4.4% as unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of ensartinib were observed based on age (20 to 86 years), sex, race (Asian vs White), body weight (38 to 148 kg), mild to moderate renal impairment (eGFR 30 to 89 mL/min) and mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and AST > ULN or total bilirubin 1 to 1.5 x ULN and any AST).
The effect of severe renal impairment (eGFR 15 to 29 mL/min), end-stage renal disease (eGFR <15 mL/min) with or without hemodialysis, and moderate (total bilirubin >1.5 to ≤ 3 ULN and any AST) or severe (total bilirubin >3 times ULN and any AST) hepatic impairment on ensartinib pharmacokinetics is unknown.
Drug Interaction Studies
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Ensartinib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and does not induce CYP1A2, CYP2B6, or CYP3A.
Transporter Systems: Ensartinib is a P-gp substrate but is not a substrate of BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1 or OCT2.
Ensartinib does not inhibit BCRP, P-gp, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2 or OCT3.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with ensartinib.
Ensartinib was not mutagenic in a bacterial reverse mutation (Ames) assay and was not clastogenic in an in vitro human lymphocyte chromosome aberration assay or an in vivo rat bone marrow micronucleus assay.
Dedicated fertility studies were not conducted with ensartinib. No adverse effects on male or female reproductive organs were observed in up to 3-month repeat-dose toxicology studies conducted in rats and dogs.
-
14 CLINICAL STUDIES
14.1 TKI-naive ALK-Positive Locally Advanced or Metastatic NSCLC (eXALT3 Study)
The efficacy of ENSACOVE was evaluated in eXALT3 (NCT02767804), an open-label, randomized, active-controlled, multicenter study in adult patients with locally advanced (stage IIIB following prior chemotherapy or chemoradiation or not amenable to curative intent therapy) or metastatic ALK-positive NSCLC. Patients were required to have ALK-positive NSCLC in tumor specimens as determined in local laboratories using immunohistochemistry (IHC), next-generation sequencing (NGS), polymerase chain reaction (PCR) or fluorescence in situ hybridization (FISH) tests. Of these patients, 85% (247/290) had centrally confirmed ALK rearrangements with the Vysis Break Apart FISH Probe Kit. Patients could have received one prior regimen of chemotherapy but could not have previously received an ALK-targeted therapy; patients with an ECOG performance status of 0, 1, or 2 were eligible. Patients with asymptomatic, untreated brain metastases who were not on corticosteroids and patients with asymptomatic, treated brain metastases who were on stable or decreasing dose of corticosteroids were eligible. Patients were required to have completed radiation therapy at least 2 weeks, or chemotherapy at least 4 weeks, prior to enrollment. Patients with leptomeningeal disease were ineligible.
Patients were randomized 1:1 to receive ENSACOVE 225 mg orally once daily or crizotinib 250 mg orally twice daily in 28-day cycles until disease progression or unacceptable toxicity. Randomization was stratified by prior chemotherapy (0 vs. 1), ECOG performance status (0 or 1 vs. 2), presence of central nervous system (CNS) metastases (yes or no), and geographic region (Asia vs. the rest of the world). Tumor assessments were performed every 8 weeks.
The main efficacy outcome measure was progression-free survival (PFS) as evaluated by Blinded Independent Central Review (BICR) according to RECIST version 1.1. The key secondary efficacy outcome measure was overall survival (OS); other secondary outcome measures included CNS response rate, time to CNS progression, and overall response rate (ORR).
A total of 290 patients were randomized to ENSACOVE (n=143) or crizotinib (n=147). The baseline demographic characteristics of the overall study population were median age 54 years (range: 25-90); 16% age > 65 years; 51% male; 56% Asian; 41% White and 1% Black; 7% Hispanic or Latino; ECOG PS 0 or 1 (95%); and 62% never smokers. Patients had Stage IIIB (8%) or Stage IV NSCLC (92%); 32% had received prior chemotherapy and 17% had received prior radiation. Baseline CNS metastases were present in 36% of the patients.
The eXALT3 study demonstrated a statistically significant improvement in PFS for patients randomized to ENSACOVE compared to patients randomized to crizotinib. The efficacy results as assessed by BICR are summarized in Table 6 and Figure 1.
Table 6: Efficacy Results for eXALT3 Study According to BICR Assessment Efficacy Parameter ENSACOVE
N=143Crizotinib
N=147Progression-free survival Number of events, n (%) 59 (41%) 80 (54%) Progressive disease, n (%) 51 (36%) 77 (52%) Death, n (%) 8 (6%) 3 (2%) Median, months (95% CI) 25.8 (21.8, NE) 12.7 (9.2, 16.6) Hazard ratio (95% CI) 0.56 (0.40, 0.79) p-valuea 0.0008 Overall response rate Overall response rate % (95% CI) 74% (66, 81) 67% (58, 74) Complete response % 12% 5% Partial response % 62% 61% Duration of response Number of responders, n 106 98 Median, months (95% CI) NE (22.0, NE) 27.3 (12.9, NE) CI = Confidence Interval, NE=not estimable, BICR = Blinded Independent Central Review
a p-value based on unstratified log-rank testFigure 1 Kaplan-Meier Plot of Progression-Free Survival by IRR from Study 301 (eXALT3)
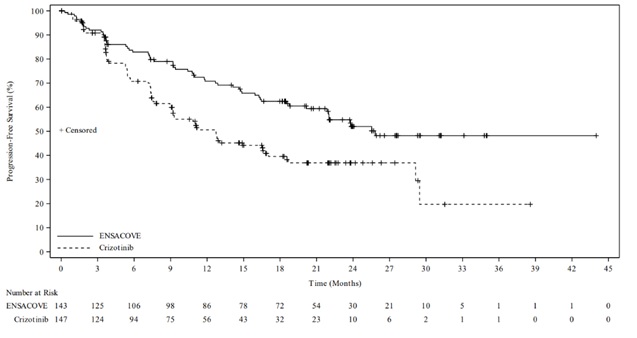
At the time of the primary PFS analysis, OS results were immature. At the time of final analysis of OS, there was no statistically significant difference (p-value = 0.4570) between ENSACOVE and crizotinib. Median OS was 63.2 months in the ENSACOVE arm and 55.7 months in the crizotinib arm, with the hazard ratio of 0.88 (95% CI: 0.63, 1.23).
The results of the pre-specified analyses of CNS response rate by BICR in patients with baseline measurable CNS disease are summarized in Table 7.
Table 7: IRR-assessed CNS Responses in Patients with Measurable CNS Disease at Baseline in Study 301 (eXALT3) Efficacy Parameter ENSACOVE
N=17crizotinib
N=24CNS overall response rate % (95% CI) 59% (33, 82) 21% (7, 42) Complete response % 24% 8% Partial response % 35% 13% Duration of Response Number of responders, n 10 5 Patients with DOR ≥ 12 months 30% 40% BICR = Blinded Independent Central Review; CI = Confidence Interval -
16 HOW SUPPLIED
ENSACOVE (ensartinib) capsules are supplied as follows:
Capsule Strength Description Package Configuration NDC Code 25 mg Size 2 capsule, white opaque cap and body, with “X-396” on the cap and “25 mg” on the body printed in blue ink.
Bottles of 30 83076-1025-3 100 mg Size 0 capsule, blue opaque cap and yellow opaque body, with “X- 396” on the cap and “100 mg” on the body printed in white ink.
Bottles of 60 83076-1100-6 Store at controlled room temperature 20ºC to 25ºC (68ºF to 77ºF); excursions permitted to 15ºC to 30ºC (59ºF to 86ºF) [see USP Controlled Room Temperature]. Store and dispense in the original bottle with desiccant to protect from moisture. Do not remove desiccant from bottle. Keep out of reach of children
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Interstitial Lung Disease (ILD)/Pneumonitis
Inform patients of the risk of severe ILD/pneumonitis during treatment with ENSACOVE. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms [see Warnings and Precautions (5.1)].
Hepatoxicity
Inform patients of the potential risk of hepatoxicity during treatment with ENSACOVE and of the need to monitor for aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bilirubin elevations during treatment with ENSACOVE. Advise patients to inform their healthcare provider of any new or worsening symptoms [see Warnings and Precautions (5.2)].
Dermatologic Adverse Reactions
Inform patients of the potential risk of dermatologic adverse reactions, including rash, pruritus, and photosensitivity during treatment with ENSACOVE. If dermatologic reactions occur, advise patients to limit sun exposure while taking ENSACOVE and for at least 1 week after the final dose [see Warnings and Precautions (5.3)].
Bradycardia
Advise patients of the risk of bradycardia during treatment with ENSACOVE and to report any symptoms of bradycardia. Advise patients to inform their healthcare provider about the use of any heart or blood pressure medications during treatment with ENSACOVE [see Warnings and Precautions (5.4)].
Hyperglycemia
Inform patients of the risks of new or worsening hyperglycemia during treatment with ENSACOVE and the need to periodically monitor glucose levels. Advise patients with diabetes mellitus or glucose intolerance that antihyperglycemic medications may need to be adjusted during treatment with ENSACOVE [see Warnings and Precautions (5.5)].
Visual Disturbances
Advise patients to inform their healthcare provider of any new or worsening vision symptoms during treatment with ENSACOVE [see Warnings and Precautions (5.6)].
Creatine Phosphokinase (CPK) Elevation
Inform patients of the signs and symptoms of creatine phosphokinase (CPK) elevation and the need for monitoring during treatment with ENSACOVE. Advise patients to inform their healthcare provider of any new or worsening symptoms [see Warnings and Precautions (5.7)].
Hyperuricemia
Inform patients of the signs and symptoms of hyperuricemia. Advise patients to inform their healthcare provider if they experience signs or symptoms associated with hyperuricemia during treatment with ENSACOVE [see Warnings and Precautions (5.8)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy. Advise females of reproductive potential to use effective contraception during treatment with ENSACOVE and for 1 week after the last dose [see Warnings and Precautions (5.9) and Use in Specific Populations (8.1,8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with ENSACOVE and for 1 week after the last dose [see Warnings and Precautions (5.9) and Use in Specific Populations (8.3)].
FD&C Yellow No. 5 (tartrazine)
Advise patients that this product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type or asthma-type reactions in certain susceptible persons (e.g., patients who also have aspirin hypersensitivity) [see Warnings and Precautions (5.10)]. Advise patients to contact their healthcare provider and seek medical help right away if they develop symptoms of an allergic reaction to FD&C Yellow No. 5 (tartrazine).
Lactation
Advise women not to breastfeed during treatment with ENSACOVE and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Administration
Instruct patients to take ENSACOVE once a day with or without food and to swallow ENSACOVE capsules whole [see Dosage and Administration (2.2)].
Missed Dose
Advise patients to take ENSACOVE at the same time each day. If a dose is missed, then they should take the missed dose as soon as possible unless the next dose is due within 12 hours. Patients should be instructed not to take 2 doses at the same time to make up for a missed dose. In addition, instruct patients not to take an extra dose if they vomit after taking ENSACOVE [see Dosage and Administration (2.2)].
Manufactured for: Xcovery Holdings, Inc. Miami, FL 33131
ENSACOVE is a trademark of Xcovery Holdings, Inc.
©2026, Xcovery Holdings, Inc. All rights reserved.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
ENSACOVETM (En-sa-kowv)
(ensartinib)
capsules, for oral useWhat is ENSACOVE? ENSACOVE is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC):
- that is caused by an abnormal anaplastic lymphoma kinase (ALK) gene,
- that has spread to other parts of your body, and
- who have not received medicines called ALK-inhibitors.
Your healthcare provider will perform a test to make sure that ENSACOVE is right for you.
It is not known if ENSACOVE is safe and effective in children.Do not use ENSACOVE if you are allergic to ENSACOVE, FD&C No. 5 (tartrazine), or to any of the ingredients in ENSACOVE. See the end of this leaflet for a complete list of ingredients in ENSACOVE.
Before using ENSACOVE, tell your healthcare provider about all of your medical conditions, including if you:
- have lung or breathing problems
- have liver problems
- have problems with your heartbeat
- have diabetes mellitus or glucose intolerance
- have problems with your vision
- are pregnant or plan to become pregnant. ENSACOVE can harm your unborn baby.
- Your healthcare provider will do a pregnancy test before you start treatment with ENSACOVE.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with ENSACOVE.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with ENSACOVE and for 1 week after the last dose of ENSACOVE. Talk to your healthcare provider about birth control choices that are right for you during treatment with ENSACOVE.
- Males who who have female partners who are able to become pregnant should use effective birth control (contraception) during treatment with ENSACOVE and for 1 week after the last dose of ENSACOVE.
- are breastfeeding or plan to breastfeed. It is not known if ENSACOVE passes into your breast milk. Do not breastfeed during treatment with ENSACOVE and for 1 week after the last dose. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the other medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Certain other medicines may affect the way that ENSACOVE works and may increase your risk of certain side effects.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take ENSACOVE? - Take ENSACOVE exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking ENSACOVE unless your healthcare provider tells you to.
- Swallow ENSACOVE capsules whole. Do not crush or chew capsules. Do not open or dissolve the contents of the capsule.
- Take ENSACOVE 1 time a day, at the same time each day.
- You may take ENSACOVE with or without food.
- If you miss a dose, take it as soon as you remember. If your next dose is due within 12 hours, skip the missed dose and take your next dose at your regular time. Do not take 2 doses of ENSACOVE on the same day to make up for the missed dose.
- If you vomit after taking a dose of ENSACOVE, do not take an extra dose. Take your next dose at your regular time.
What should I avoid while taking ENSACOVE? - Limit your time in the sun during treatment with ENSACOVE and for at least 1 week after your last dose. ENSACOVE may make your skin sensitive to sunlight. You may burn more easily and get severe sunburns. When you are in the sun, wear a hat and protective clothing, and use a broad-spectrum sunscreen and lip balm with a Sun Protection Factor (SPF) of 30 or greater to protect against sunburn.
What are the possible side effects of ENSACOVE? ENSACOVE can cause serious side effects, including:
- Lung problems. ENSACOVE can cause severe or life-threatening swelling (inflammation) of the lungs. Symptoms may be similar to those from lung cancer. Tell your healthcare provider right away if you get any new or worsening symptoms of lung problems during treatment with ENSACOVE, including:
- trouble breathing or shortness or breath
- chest pain
- cough with or without mucus
- fever
- Liver problems. ENSACOVE can increase enzymes called aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and levels of bilirubin in your blood. Tell your healthcare provider if you get new or worsening signs or symptoms of liver problems, including:
- yellowing of your skin or the white part of your eyes
- dark or brown (tea color) urine
- nausea or vomiting
- pain on the right side of your stomach area
- bleed or bruise more easily than normal
- itchy skin
- decreased appetite
- feeling tired
- Skin reactions. ENSACOVE may cause skin reactions that require treatment. Tell your healthcare provider if you get symptoms of skin reactions, such as rash, itching, or skin swelling
- Slow heart rate (bradycardia). ENSACOVE can cause very slow heartbeats that can be severe. Your healthcare provider will check your heart rate during treatment with ENSACOVE. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint during treatment with ENSACOVE. Tell your healthcare provider if you take any heart or blood pressure medicines.
- High blood sugar (hyperglycemia). ENSACOVE can increase your blood sugar levels. Hyperglycemia is common with ENSACOVE treatment but can be serious. If you take medicine for diabetes or glucose intolerance your healthcare provider may change your medicine during treatment with ENSACOVE. Tell your healthcare provider if you get new or worsening signs and symptoms of hyperglycemia, including:
- feeling very thirsty
- needing to urinate more than usual
- feeling very hungry
- feeling sick to your stomach
- feeling weak or tired
- feeling confused
- Vision problems. ENSACOVE may cause vision problems. Your healthcare provider may refer you to an eye specialist if you develop new or worsening vision problems during treatment with ENSACOVE. Tell your healthcare provider if you have any loss of vision or any change in vision, including
- blurry vision
- double vision
- seeing flashes of light
- light hurting eyes
- new or increased floaters
- Muscle pain, tenderness, and weakness (myalgia). ENSACOVE can increase the level of an enzyme in your blood called creatine phosphokinase (CPK), which may be a sign of muscle damage. Myalgia is common with ENSACOVE treatment but can be serious. Tell your healthcare provider if you get new or worsening signs and symptoms of muscle problems, including unexplained muscle pain or muscle pain that does not go away, tenderness, or weakness.
- Increased uric acid level in your blood (hyperuricemia). ENSACOVE can cause too much uric acid in your blood. Hyperuricemia is common with ENSACOVE treatment but can be serious. Your healthcare provider may prescribe medicines if you have high blood uric acid levels. Tell your healthcare provider if you develop any of the following symptoms of hyperuricemia:
- red, hot, tender, or swollen joints, especially your big toe
- pain in your stomach-area or sides
- nausea or vomiting
- pink or brown urine
- Allergic reactions to FD&C Yellow No. 5 (tartrazine). ENSACOVE 100 mg capsules contain FD&C Yellow No. 5. FD&C Yellow No. 5 (tartrazine) that can cause allergic reactions in certain people, especially people who also have an allergy to aspirin. Tell your healthcare provider if you get hives, rash, or trouble breathing during treatment with ENSACOVE.
Your healthcare provider will do certain blood tests before and during treatment with ENSACOVE to check you for side effects.
If you have serious side effects during treatment with ENSACOVE, your healthcare provider may change your dose, stop your treatment for a period of time (temporary), or completely stop treatment with ENSACOVE.
The most common side effects of ENSACOVE are:
- rash
- muscle or bone pain
- constipation
- itching
- coughing
- nausea
- skin swelling (edema)
- vomiting
- tiredness
- fever
- increased levels of liver and pancreatic enzymes
- decreased white blood cell counts
- changes in blood levels of phosphate, magnesium, sodium, and potassium
- decreased protein (hemoglobin) in red blood cells
- increased bilirubin blood levels
These are not all the possible side effects with ENSACOVE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store ENSACOVE? - Store ENSACOVE at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Keep ENSACOVE capsules in the original bottle.
- The bottle of ENSACOVE capsules contains a drying agent (desiccant) to help keep your medicine dry. Do not remove the desiccant from the bottle after opening. Do not open or eat the desiccant.
Keep ENSACOVE and all medicines out of the reach of children.
General information about the safe and effective use of ENSACOVE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ENSACOVE for a condition for which it was not prescribed. Do not give ENSACOVE to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ENSACOVE that is written for health professionals.What are the ingredients in ENSACOVE? Active ingredient: ensartinib hydrochloride
Inactive ingredients: butyl alcohol, dehydrated alcohol, hypromellose, isopropyl alcohol, microcrystalline cellulose, propylene glycol, shellac, stearic acid, and titanium dioxide. The 25 mg capsules also contain the following inactive ingredients: FD&C Blue No. 2 and strong ammonia solution. The 100 mg capsules also contain the following inactive ingredients: black iron oxide, FD&C Blue No. 1, FD&C Yellow No. 5, povidone, red iron oxide, and sodium hydroxide.
Manufactured for: Xcovery Holdings, Inc., Miami, FL, 33131
For more information, call Xcovery Holdings, Inc. at (866) 367-2268.
ENSACOVE is a trademark of Xcovery Holdings, Inc.
©2024, Xcovery Holdings, Inc. All rights reserved.
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 12/2024 - PRINCIPAL DISPLAY PANEL - 25 mg
- PRINCIPAL DISPLAY PANEL - 100 mg
-
INGREDIENTS AND APPEARANCE
ENSACOVE
ensartinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83076-1025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSARTINIB HYDROCHLORIDE (UNII: C2FR6VT1BQ) (ENSARTINIB - UNII:SMA5ZS5B22) ENSARTINIB HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SHELLAC (UNII: 46N107B71O) ISOPROPYL ALCOHOL (UNII: ND2M416302) DEHYDRATED ALCOHOL (UNII: 3K9958V90M) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color white (opaque;body;cap) Score no score Shape OVAL Size 18mm Flavor Imprint Code X396;25;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83076-1025-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218171 04/30/2025 ENSACOVE
ensartinib capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83076-1100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ENSARTINIB HYDROCHLORIDE (UNII: C2FR6VT1BQ) (ENSARTINIB - UNII:SMA5ZS5B22) ENSARTINIB 100 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SHELLAC (UNII: 46N107B71O) ISOPROPYL ALCOHOL (UNII: ND2M416302) DEHYDRATED ALCOHOL (UNII: 3K9958V90M) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) POVIDONE (UNII: FZ989GH94E) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color blue (opaque;cap) , yellow (opaque;body) Score no score Shape OVAL Size 22mm Flavor Imprint Code X396;100;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83076-1100-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218171 04/30/2025 Labeler - Xcovery Holdings, Inc. (033440609)
Trademark Results [ENSACOVE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENSACOVE 97812894 not registered Live/Pending |
Xcovery Holdings, Inc. 2023-02-27 |
 ENSACOVE 88409302 not registered Live/Pending |
Xcovery Holdings, Inc. 2019-04-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.