Treatonic Tattoo Numbing Cream by Shenzhen Situya Trading Co., Ltd. 82739-021 Complete
Treatonic Tattoo Numbing Cream by
Drug Labeling and Warnings
Treatonic Tattoo Numbing Cream by is a Otc medication manufactured, distributed, or labeled by Shenzhen Situya Trading Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
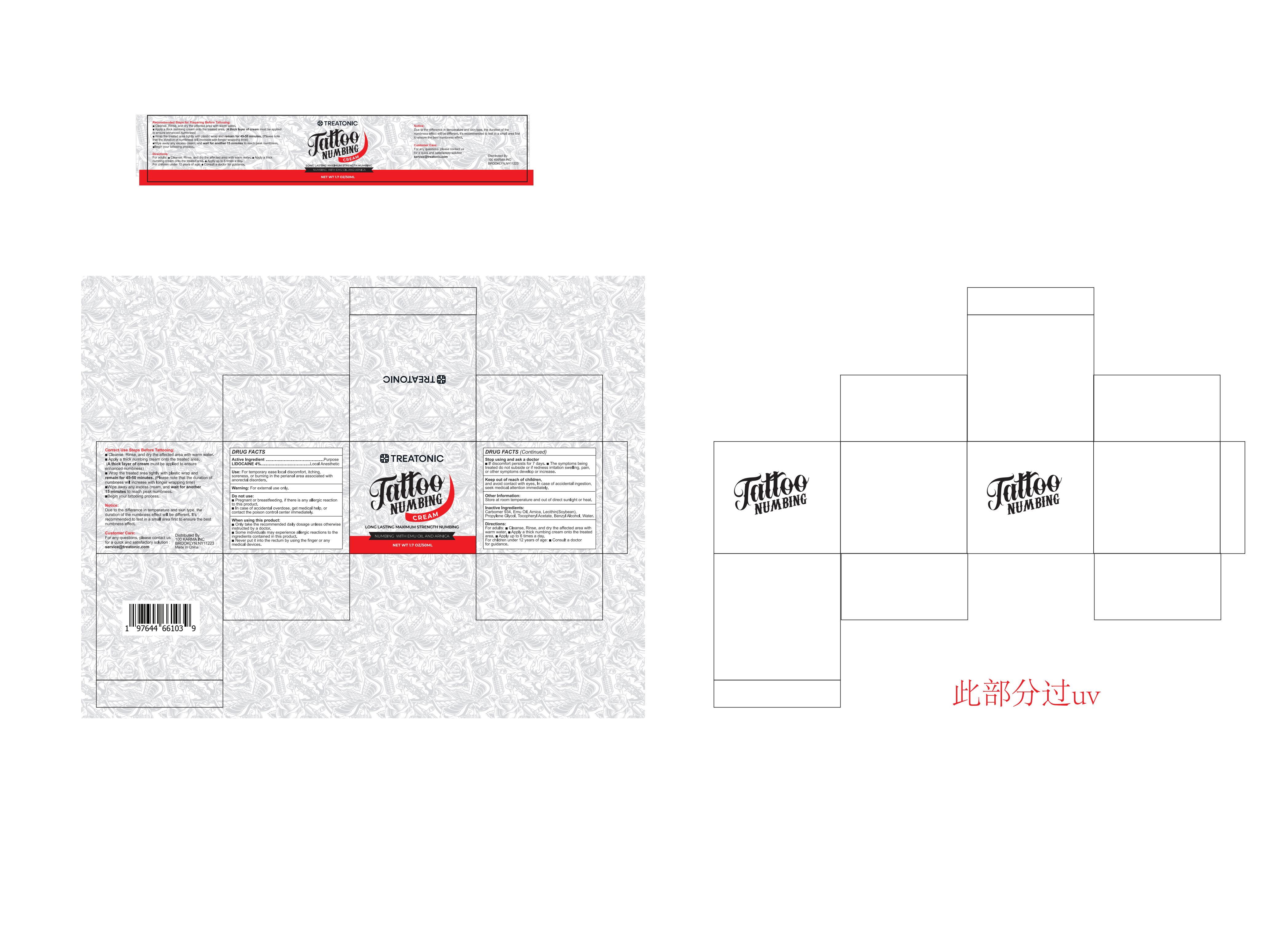
TREATONIC TATTOO NUMBING CREAM- tattoo numbing cream cream
Shenzhen Situya Trading Co., Ltd.
----------
82739-021 Complete
Use
For temporary ease local discomfort, itching, soreness, or burning in the perianal area associated with anorectal disorders.
Do not use
■ Pregnant or breastfeeding, if there is any allergic reaction to this product.
■ In case of accidental overdose, get medical help, or contact the poison control center immediately.
When Using
■ Only take the recommended daily dosage unless otherwise instructed by a doctor.
■ Some individuals may experience allergic reactions to the ingredients contained in this product.
■ Never put it into the rectum by using the finger or any medical devices.
Ask Doctor
■ if discomfort persists for 7 days.
■ The symptoms being treated do not subside or if redness irritation swelling, pain, or other symptoms develop or increase.
Keep Oot Of Reach Of Children
and avoid contact with eyes.
In case of accidental ingestion, seek medical attention immediately
Directions
For adults:
■ Cleanse, Rinse, and dry the affected area with warm water.
■ Apply a thick numbing cream onto the treated area.
■ Apply up to 6 times a day.
For children under 12 years of age:
■ Consult a doctor for guidance.
| TREATONIC TATTOO NUMBING CREAM
tattoo numbing cream cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Situya Trading Co., Ltd. | 706154255 | label(82739-021) , manufacture(82739-021) | |