MATRIX- altrenogest solution
Matrix by
Drug Labeling and Warnings
Matrix by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp., Intervet Production S.A., Aspen Oss B.V., Euroapi France. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- VETERINARY INDICATIONS
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
WARNINGS
WARNINGS:
User/Handler Safety:
Keep this and all medication out of the reach of children.
Avoid skin contact. Wear vinyl, neoprene or nitrile protective gloves when handling this product. DO NOT USE LATEX GLOVES Pregnant women or women who suspect they are pregnant should not handle MATRIX® (altrenogest) Solution 0.22%. Women of childbearing age should exercise extreme caution when handling this product. Accidental absorption could lead to a disruption of the menstrual cycle or prolongation of pregnancy. Wash off accidental spillage on the skin immediately with soap and water.
People who should not handle this product:
- Women who are or suspect they are pregnant.
- Anyone with thrombophlebitis or thromboembolic disorders or with a history of these events.
- Anyone with cerebral-vascular or coronary-artery disease.
- Women with known or suspected carcinoma of the breast.
- People with known or suspected estrogen-dependent neoplasia.
- Women with undiagnosed vaginal bleeding.
- People with benign or malignant tumors which developed during the use of oral contraceptives or other estrogen-containing products.
- Anyone with liver dysfunction or disease.
Accidental exposure: Altrenogest is readily absorbed from contact with the skin. In addition, this oil based product can penetrate porous gloves. Altrenogest should not penetrate intact vinyl, neoprene or nitrile protective gloves; however, if there is leakage (i.e., pinhole, spillage, etc.) the contaminated area covered by such occlusive materials may have increased absorption. DO NOT USE LATEX GLOVES
The following measures are recommended in case of accidental exposure.
Skin Exposure: Wash immediately with soap and water.
Eye Exposure: Immediately flush with plenty of water for 15 minutes. Get medical attention.
If Swallowed: Do not induce vomiting. MATRIX® (altrenogest) Solution 0.22% contains an oil. Call a physician. Vomiting should be supervised by a physician because of possible pulmonary damage via aspiration of the oil base. If possible, bring the container and labeling to the physician.
Effects of Overexposure: There has been no human use of this specific product. The information contained in this section is extrapolated from data available on other products of the same pharmacological class that have been used in humans. Effects anticipated are due to the progestational activity of altrenogest. Acute effects after a single exposure are possible; however, continued daily exposure has the potential for more untoward effects such as disruption of the menstrual cycle, uterine or abdominal cramping, increased or decreased uterine bleeding, prolongation of pregnancy and headaches. The oil base may also cause complications if swallowed. In addition, the list of people who should not handle this product is based upon the known effects of progestins used in humans on a chronic basis.
Human Food Safety: Gilts must not be slaughtered for human consumption for 21 days after the last treatment.
- SPL UNCLASSIFIED SECTION
-
DOSAGE & ADMINISTRATION
Dosage and Directions: While wearing protective gloves, remove shipping cap and seal; replace with enclosed plastic dispensing cap. Connect the Matrix® Dosing Device to the solution bottle according to the dosing device instructions provided as an attachment to the Matrix® Dosing Device package.
Administer 6.8 mL (15 mg altrenogest) per gilt once daily for 14 consecutive days. Treat gilts on an individual animal basis by top-dressing MATRIX® on a portion of each gilt's daily feed allowance. To produce the desired synchronization of estrus in a group of gilts, treat all of the gilts daily for the same 14-day period.
- SPL UNCLASSIFIED SECTION
-
Questions? Comments?
- To report adverse reaction call Merck at 1-800-211-3573
- To obtain product information, including material safety data sheet (MSDS), call 1-800-441-8272.
- For additional information about adverse drug experience for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/Animal/Veterinary/SafetyHealth
- SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR USE
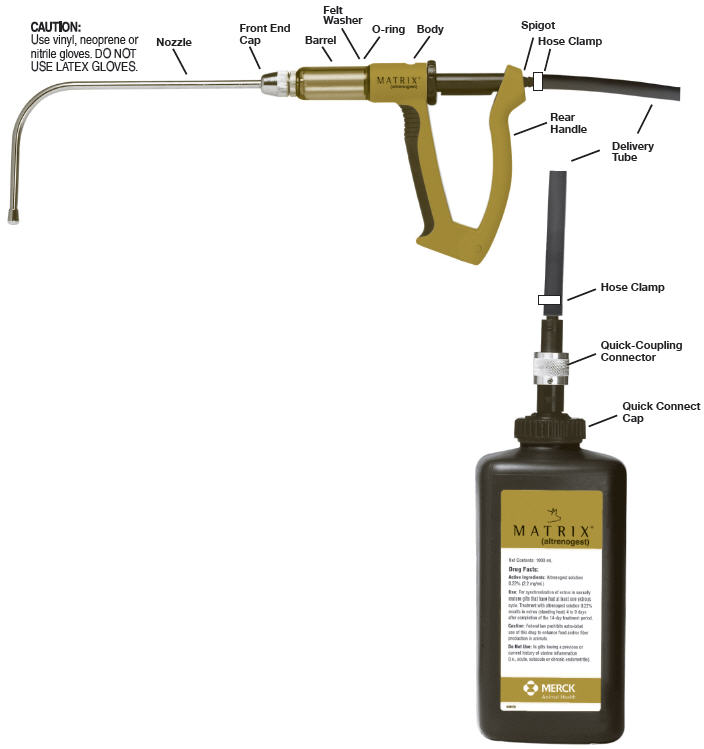
Dosing Gun (for top dressing of swine feed)
for use with
MATRIX® (altrenogest)NOZZLE FITTING INSTRUCTIONS
- Connect nozzle and cap to barrel and screw on 7/8 of the way.
- Position nozzle at preferred angle, then tighten cap fully.
Contain any spillage with absorbent materials such as towels. Place empty drug containers, protective gloves or other articles that come in contact with MATRIX® in a leak-resistant container for disposal in accordance with applicable federal, state and local regulations.
OPERATING INSTRUCTIONS
Use vinyl, neoprene or nitrile gloves while using or cleaning the gun and gun parts. DO NOT USE LATEX GLOVES.
- Remove the shipping cap and seal on MATRIX® (altrenogest) bottle. Store the cap in a clean and dry location.
- Apply downward force and fasten the quick connect cap with dip tube onto the product bottle. (The flexible dip tube will contact the bottom of the product bottle and bend slightly)
- Use the black delivery tubing to connect the barbed connectors on the spigot and on the quick–coupling connector.
- Clamp the tubing at the spots shown on the diagram.
Connect the quick-coupling connector to the quick connect cap. - Hold the dosing gun vertically with the nozzle on the top. Direct the opening of the nozzle away from any person and cover it with absorbent material. Slowly squeeze and release the handle until air in the barrel is expelled and product starts to come out.
- Prime the gun by expelling two (2) doses of product into a waste container or absorbent material. (Re-prime the gun by expelling two (2) doses if air is observed inside the barrel after switching to a fresh bottle or during dosing period)
- The gun is ready for dosing.
- Store the dosing gun when loaded with product for continued use at or below room temperature, 77 °F ( 25 °C).
CLEANING INSTRUCTIONS
Use vinyl, neoprene or nitrile gloves while using or cleaning the gun and gun parts. DO NOT USE LATEX GLOVES.
- The dosing gun should be disassembled and cleaned following the below instruction after every 14-day dosing period and does not need to be cleaned daily while in use.
- Unscrew the dosing cap and replace shipping cap onto the product bottle to prevent spillage.
- Place the dip tube attached on the quick connect cap into hot soapy water and flush out residual product.
- Flush with clean water. Expel the water by flushing air through.
- Remove the barrel from the dosing gun body and dry the O-ring and felt washer. Apply an adequate amount (2-3 drops) of vegetable oil with a gloved finger to coat the O-ring and felt washer. (DO NOT USE MINERAL OIL)
- Reattach the barrel to dosing gun body and store the whole assembly in a cool and dry place.
- If squeezing the dosing gun handle to dispense product becomes increasingly difficult, the O-ring and felt washer may need to be replaced.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1000 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
MATRIX
altrenogest solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 57926-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength altrenogest (UNII: 2U0X0JA2NB) (altrenogest - UNII:2U0X0JA2NB) altrenogest 2.2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57926-101-70 1000 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141222 09/12/1983 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [Matrix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MATRIX 98756196 not registered Live/Pending |
Infonet Corp. 2024-09-18 |
 MATRIX 98605432 not registered Live/Pending |
FNTE INTERNATIONAL CO., LTD. 2024-06-17 |
 MATRIX 98535181 not registered Live/Pending |
Matrix Pointe Software LLC 2024-05-06 |
 MATRIX 98535172 not registered Live/Pending |
Matrix Pointe Software LLC 2024-05-06 |
 MATRIX 98305293 not registered Live/Pending |
The Marine Group, LLC 2023-12-08 |
 MATRIX 98274969 not registered Live/Pending |
Matrix Arms & XRAY Aerospace Corp 2023-11-17 |
 MATRIX 98242846 not registered Live/Pending |
Flexstar Inc 2023-10-26 |
 MATRIX 98242842 not registered Live/Pending |
Flexstar Inc 2023-10-26 |
 MATRIX 98242837 not registered Live/Pending |
Flexstar Inc 2023-10-26 |
 MATRIX 98129191 not registered Live/Pending |
BMIC LLC 2023-08-11 |
 MATRIX 98030945 not registered Live/Pending |
MAPLE TATTOO SUPPLY INC. 2023-06-07 |
 MATRIX 98012470 not registered Live/Pending |
Infonet Corp. 2023-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
