Lidozo Topical Pain Patch
Lidozo Topical Pain Patch by
Drug Labeling and Warnings
Lidozo Topical Pain Patch by is a Otc medication manufactured, distributed, or labeled by GREENHILL TRADING, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIDOZO TOPICAL PAIN PATCH- lidocaine 4% patch
GREENHILL TRADING, INC.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
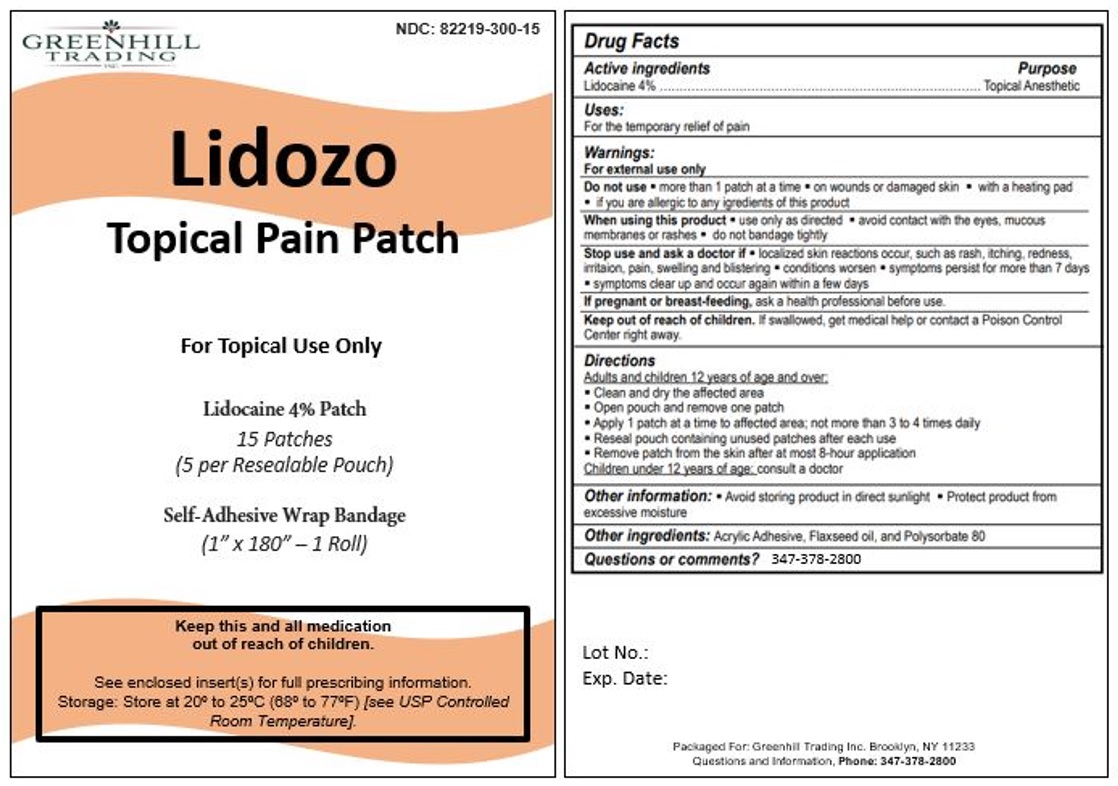
Lidozo Topical Pain Patch
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes, or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- conditions worsen
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age or over:
- Clean and dry the affected area
- Open pouch and remove one patch
- Apply 1 patch at a time to affected area; not more than 3 to 4 times daily
- Reseal pouch containing unused patches after each use
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
Questions or comments? 347-378-2800
Lidozo
Topical Pain Patch
NDC: 82219-300-15
For Topical Use Only
Lidocaine 4% Patch
15 Patches
(5 per Resealable Pouch)
Self-Adhesive Wrap Bandage
(1" X 180" - 1 Roll)
Manufactured for:
GREENHILL
TRADING
| LIDOZO TOPICAL PAIN PATCH
lidocaine 4% patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GREENHILL TRADING, INC. (965879054) |