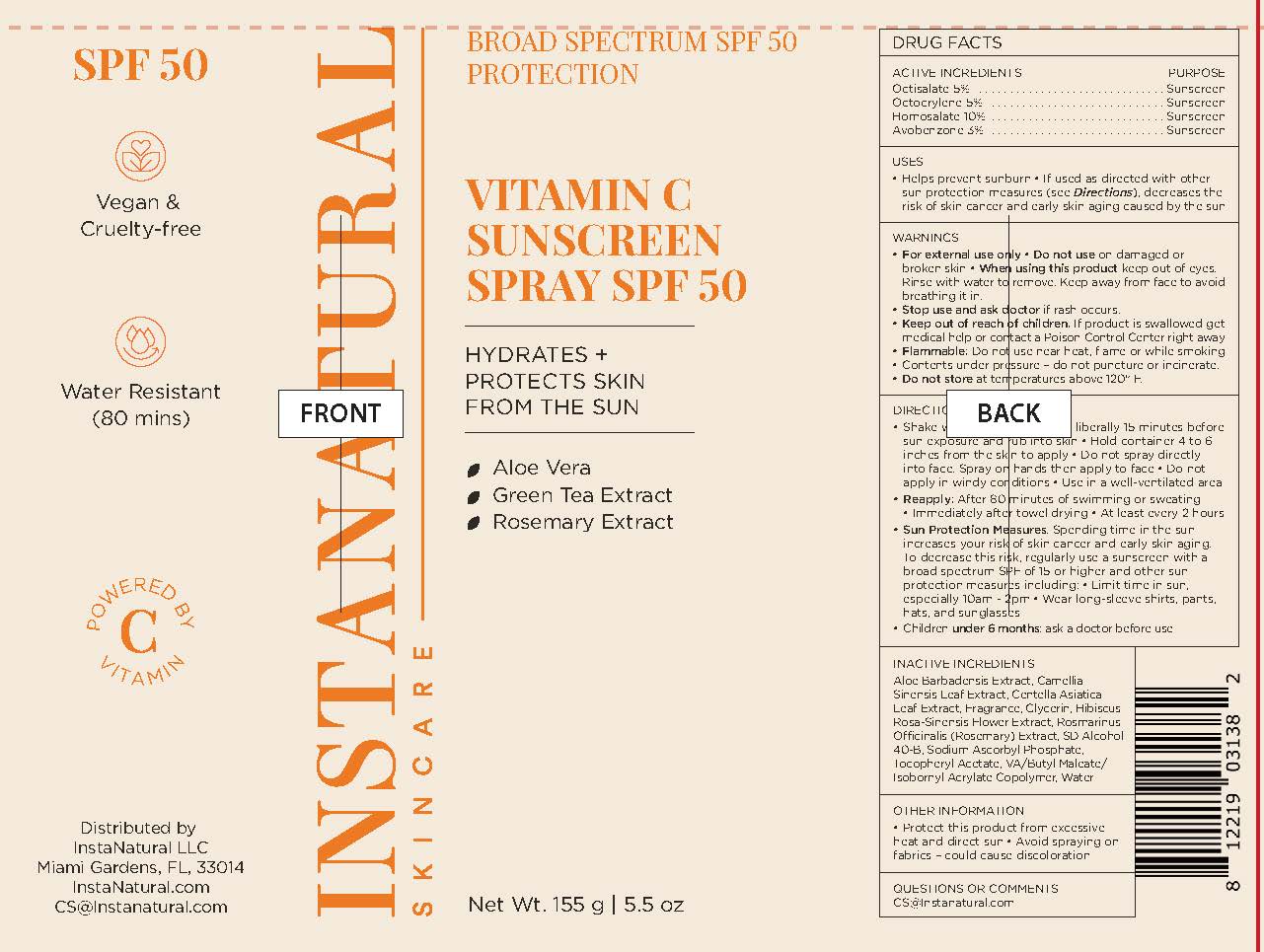
Instanatural Vitamin C Sunscreen Spray SPF 50
Instanatural by
Drug Labeling and Warnings
Instanatural by is a Otc medication manufactured, distributed, or labeled by Prime Packaging, Prime Enterprises. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
INSTANATURAL BROAD SPECTRUM SPF 50- octisalate, octocrylene, homosalate, avobenzone aerosol
Prime Packaging
----------
Instanatural Vitamin C Sunscreen Spray SPF 50
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
- For external use only
- Flammable: Do not use near heat, flame or while smoking
- Contents under pressure - do not puncture or incinerate
- Do not store at temperatures above 120F
Directions
- Shake well
- Spray liberally 15 minutes before sun exposure and rub into skin
- Hold container 4 to 6 inches from the skin to apply
- Do not spray directly into face. Spray on hands then apply to face.
- Do not apply in windy conditions
- Use in a well-ventilated area
- Reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in sun, especailly 10am - 2pm
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: ask a doctor before use
Inactive Ingredients
Aloe Barbadensis Extract, Camellia Sinensis Leaf Extract, Centella Asiatica Leaf Extract, Fragrance, Glycerin, Hibiscus Rosa-Sinensis Flower Extract, Rosmarinus Officinalis (Rosemary) Extract, SD Alcohol 40-B, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, VA/Butyl Maleate/Isobornyl Acrylate Copolymer, Water
| INSTANATURAL
BROAD SPECTRUM SPF 50
octisalate, octocrylene, homosalate, avobenzone aerosol |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Prime Packaging (805987059) |
| Registrant - Prime Packaging (805987059) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Prime Packaging | 805987059 | label(13630-0289) , pack(13630-0289) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Prime Enterprises | 101946028 | analysis(13630-0289) , manufacture(13630-0289) | |
Revised: 7/2024
Document Id: 1e923040-a8d2-dd6f-e063-6294a90a6a67
Set id: 1e923040-a8d1-dd6f-e063-6294a90a6a67
Version: 1
Effective Time: 20240731
Trademark Results [Instanatural]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INSTANATURAL 88946196 not registered Live/Pending |
InstaNatural, LLC 2020-06-03 |
 INSTANATURAL 87189532 5204260 Live/Registered |
InstaNatural, LLC 2016-09-30 |
 INSTANATURAL 86655090 4877723 Live/Registered |
InstaNatural, LLC 2015-06-08 |
 INSTANATURAL 86272144 not registered Dead/Abandoned |
InstaNatural 2014-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.