ESOMEPRAZOLE MAGNESIUM capsule, delayed release
Esomeprazole Magnesium by
Drug Labeling and Warnings
Esomeprazole Magnesium by is a Prescription medication manufactured, distributed, or labeled by Advanced Rx of Tennessee, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
Esomeprazole Magnesium DR Capsules
(Source Stock Size 90 Count)
These highlights do not include all the information needed to use ESOMEPRAZOLE MAGNESIUM DELAYED-RELEASE CAPSULES safely and effectively. See full prescribing information for ESOMEPRAZOLE MAGNESIUM DELAYED-RELEASE CAPSULES.
ESOMEPRAZOLE MAGNESIUM delayed-release capsules, for oral use
Initial U.S. Approval: 1989 (omeprazole)INDICATIONS AND USAGE
Esomeprazole magnesium delayed-release capsules are a proton pump inhibitor (PPI).
Esomeprazole magnesium delayed-release capsules are indicated for the:
· Short-term treatment in the healing of erosive esophagitis (EE) in adults and pediatric patients 12 years to 17 years of age. ( 1.1)
· Maintenance of healing of EE in adults. ( 1.2)
· Short-term treatment of heartburn and other symptoms associated GERD in adults and pediatric patients 12 years to 17 years of age. ( 1.3)
· Risk reduction of nonsteroidal anti-inflammatory drugs (NSAID)-associated gastric ulcer in adults at risk for developing gastric ulcers due to age (60 years and older) and/or documented history of gastric ulcers. ( 1.4)
· Helicobacter pylori eradication in adult patients to reduce the risk of duodenal ulcer recurrence in combination with amoxicillin and clarithromycin. ( 1.5)
· Long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome in adults. ( 1.6)
DOSAGE AND ADMINISTRATION
Population
Recommended Adult (2.1) and Pediatric Dosage (2.2)
Healing of EE (1 year and older)
EE due to Acid-Mediated GERD (1 month to less than 1 year)
Adults
20 mg or 40 mg 1 once daily for 4 to 8 weeks; some patients may require an additional 4 to 8 weeks
12 years to 17 years
20 mg or 40 mg 1 once daily for 4 to 8 weeks
Maintenance of Healing of EE
Adults
20 mg once daily. Controlled studies do not extend beyond 6 months
Treatment of Symptomatic GERD
Adults
20 mg once daily once daily for 4 weeks some patients may require an additional 4 weeks
12 years to 17 years
20 mg once daily for 4 weeks
Risk Reduction of NSAID-Associated Gastric Ulcer
Adults
20 mg or 40 mg 1 once daily for up to 6 months 2
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Adults
Esomeprazole magnesium delayed-release capsules 40 mg 1once daily for 10 days
Amoxicillin 1000 mg twice daily for 10 days 3
Clarithromycin 500 mg twice daily for 10 days 3
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
Adults
Starting dosage is 40 mg twice daily 4 (varies with the individual patient) as long as clinically indicated.
1A maximum dosage of 20 mg once daily is recommended for patients with severe liver impairment (Child-Pugh Class C).
2Controlled studies do not extend beyond 6 months.
3Refer to the amoxicillin and clarithromycin prescribing information for dosage adjustments in elderly and renally-impaired patients.
4A starting dosage of 20 mg twice daily is recommended for patients with severe liver impairment (Child-Pugh Class C).
Preparation and Administration Information
DOSAGE FORMS AND STRENGTHS
· Esomeprazole Magnesium Delayed-Release Capsules: 20 mg and 40 mg. ( 3)
CONTRAINDICATIONS
· Known hypersensitivity to substituted benzimidazoles or any component of the formulation. ( 4)
· Patients receiving rilpivirine-containing products. ( 4, 7)
· Refer to the Contraindications section of the prescribing information for amoxicillin and clarithromycin, when administered in combination with esomeprazole magnesium delayed-release capsules. ( 4)
WARNINGS AND PRECAUTIONS
· Gastric Malignancy: In adults, symptomatic response does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing. (5.1)
· Acute Tubulointerstitial Nephritis: Discontinue treatment and evaluate patients. (5.2)
· Clostridium difficile-Associated Diarrhea: PPI therapy may be associated with increased risk. (5.3)
· Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4)
· Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. ( 5.5)
· Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue esomeprazole magnesium and refer to specialist for evaluation. (5.6)
· Interaction with Clopidogrel: Avoid concomitant use of esomeprazole magnesium. (5.7)
· Cyanocobalamin (Vitamin B-12) Deficiency: Daily long-term use (e.g., longer than 3 years) may lead to malabsorption or a deficiency of cyanocobalamin. (5.8)
· Hypomagnesemia and Mineral Metabolism: Reported rarely with prolonged treatment with PPIs. (5.9)
· Interaction with St. John’s Wort or Rifampin: Avoid concomitant use of esomeprazole magnesium. (5.10, 7)
· Interactions with Diagnostic Investigations for Neuroendocrine Tumors: Increased chromogranin A (CgA) levels may interfere with diagnostic investigations for neuroendocrine tumors, temporarily stop esomeprazole magnesium at least 14 days before assessing CgA levels. (5.11, 12.2)
· Interaction with Methotrexate: Concomitant use with PPIs may elevate and/or prolong serum concentrations of methotrexate and/or its metabolite, possibly leading to toxicity. With high dose methotrexate administration, consider temporary withdrawal of esomeprazole magnesium. (5.12, 7)
· Fundic Gland Polyps: Risk increases with long-term use, especially beyond one year. Use the shortest duration of therapy. (5.13)
ADVERSE REACTIONS
Most common adverse reactions (6.1):
· Adults(≥ 18 years) ( >1%) are: headache, diarrhea, nausea, flatulence, abdominal pain, constipation, and dry mouth.
· Pediatrics(1 to 17 years) ( >2%) are: headache, diarrhea, abdominal pain, nausea, and somnolence.
To report SUSPECTED ADVERSE REACTIONS, contact Hetero Labs Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for a list of clinically important drug interactions. ( 7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Healing of Erosive Esophagitis (EE)
1.2 Maintenance of Healing of EE
1.3 Treatment of Symptomatic GERD
1.4 Risk Reduction of Nonsteroidal Anti-Inflammatory Drugs (NSAID)-Associated Gastric Ulcer
1.5 Helicobacter pyloriEradication to Reduce the Risk of Duodenal Ulcer Recurrence
1.6 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults by Indication
2.2 Recommended Dosage in Pediatric Patients by Indication
2.3 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
5.2 Acute Tubulointerstitial Nephritis
5.3 Clostridium difficile-Associated Diarrhea
5.4 Bone Fracture
5.5 Severe Cutaneous Adverse Reactions
5.6 Cutaneous and Systemic Lupus Erythematosus
5.7 Interaction with Clopidogrel
5.8 Cyanocobalamin (Vitamin B-12) Deficiency
5.9 Hypomagnesemia and Mineral Metabolism
5.10 Interaction with St. John’s Wort or Rifampin
5.11 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
5.12 Interaction with Methotrexate
5.13 Fundic Gland Polyps
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Healing of EE in Adults
14.2 Maintenance of Healing of EE in Adults
14.3 Symptomatic GERD in Adults
14.4 Pediatric GERD
14.5 Risk Reduction of NSAID-Associated Gastric Ulcer
14.6 H. pyloriEradication in Adult Patients with Duodenal Ulcer Disease
14.7 Pathological Hypersecretory Conditions, Including Zollinger-Ellison Syndrome, in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Healing of Erosive Esophagitis (EE)
Adults
Esomeprazole magnesium delayed-release capsules are indicated for the short-term treatment (4 to 8 weeks) in the healing and symptomatic resolution of diagnostically confirmed EE in adults. For those patients who have not healed after 4 to 8 weeks of treatment, an additional 4- to 8- week course of esomeprazole magnesium delayed-release capsules may be considered.
Pediatric Patients 12 Years to 17 Years of Age
Esomeprazole magnesium delayed-release capsules are indicated for the short-term treatment (4 to 8 weeks) for the healing of EE in pediatric patients 12 years to 17 years of age.
1.2 Maintenance of Healing of EE
Esomeprazole magnesium delayed-release capsules are indicated for the maintenance of healing of EE in adults. Controlled studies do not extend beyond 6 months.
1.3 Treatment of Symptomatic GERD
Adults
Esomeprazole magnesium delayed-release capsules are indicated for short-term treatment (4 to 8 weeks) of heartburn and other symptoms associated with GERD in adults.
Pediatric Patients 12 Years to 17 Years of Age
Esomeprazole magnesium delayed-release capsules are indicated for short-term treatment (4 weeks) of heartburn and other symptoms associated with GERD in pediatric patients 12 years to 17 years of age.
1.4 Risk Reduction of Nonsteroidal Anti-Inflammatory Drugs (NSAID)-Associated Gastric Ulcer
Esomeprazole magnesium delayed-release capsules are indicated for the reduction in the occurrence of gastric ulcers associated with continuous NSAID therapy in adult patients at risk for developing gastric ulcers. Patients are considered to be at risk due to their age (60 years and older) and/or documented history of gastric ulcers. Controlled studies do not extend beyond 6 months.
1.5 Helicobacter pyloriEradication to Reduce the Risk of Duodenal Ulcer Recurrence
Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence.
Triple Therapy
Esomeprazole magnesium delayed-release capsules in combination with amoxicillin and clarithromycin is indicated for the treatment of adult patients with H. pylori infection and duodenal ulcer disease (active or history of within the past 5 years) to eradicate H. pylori.
In patients who fail therapy, susceptibility testing should be done. If resistance to clarithromycin is demonstrated or susceptibility testing is not possible, alternative antimicrobial therapy should be instituted [see Clinical Pharmacology ( 12.4) and the prescribing information for clarithromycin].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage in Adults by Indication
Table 1 shows the recommended adult dosage of esomeprazole magnesium delayed-release capsules by indication.
The duration of esomeprazole magnesium delayed-release capsules treatment should be based on available safety and efficacy data specific to the defined indication and dosing frequency and individual patient medical needs. Esomeprazole magnesium delayed-release capsules should only be initiated and continued if the benefits outweigh the risks of treatment.
Table 1: Recommended Dosage of Esomeprazole Magnesium Delayed-Release Capsules in Adults by Indication
Adult Indication
Recommended Dosage of
Esomeprazole Magnesium Delayed-Release Capsules
Treatment Duration
Healing of EE
20 mg or 40 mg 1 once daily
4 to 8 weeks 2
Maintenance of Healing of EE
20 mg once daily
Controlled studies do not extend beyond 6 months
Treatment of Symptomatic GERD
20 mg once daily
4 weeks; if symptoms do not resolve completely, consider an additional 4 weeks
Risk Reduction of NSAID-Associated Gastric Ulcer
20 mg or 40 mg 1once daily
Controlled studies do not extend beyond 6 months
H. pyloriEradication to Reduce the Risk of Duodenal Ulcer Recurrence (Triple Therapy)
Esomeprazole magnesium delayed-release capsules 40 mg once daily 1
10 days
Amoxicillin 1000 mg twice daily 3
10 days
Clarithromycin 500 mg twice daily 3
10 days
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome
Starting dosage is 40 mg twice daily 4; individualize the regimen to patient needs.
Dosages of up to 240 mg/day have been administered [see Clinical Studies (14.7)].
As long as clinically indicated
1.A maximum dosage of 20 mg once daily is recommended for patients with severe liver impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].
2.Most patients are healed within 4 to 8 weeks. For patients who do not heal after 4 to 8 weeks, an additional 4 to 8 weeks of treatment may be required to achieve healing [see Clinical Studies (14.1)].
3.Refer to the amoxicillin and clarithromycin prescribing information for dosage adjustments in elderly and renally-impaired patients.
4.A starting dosage of 20 mg twice daily is recommended for patients with severe liver impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].2.2 Recommended Dosage in Pediatric Patients by Indication
Table 2 shows the recommended dosage of esomeprazole magnesium delayed-release capsules in pediatric patients by indication.
Table 2: Recommended Dosage of Esomeprazole Magnesium Delayed-Release Capsules in Pediatric Patients by Indication
Indication
Patient Age
Recommended Dosage
Duration
Healing of EE
12 years to 17 years
Esomeprazole magnesium delayed-release capsules:
20 mg or 40 mg once daily
4 to 8 Weeks
Treatment of Symptomatic GERD
12 years to 17 years
Esomeprazole magnesium delayed-release capsules:
20 mg once daily
4 weeks
2.3 Preparation and Administration Instructions
- Take esomeprazole magnesium delayed-release capsules at least one hour before meals [see Clinical Pharmacology ( 12.3) ].
- Antacids may be used concomitantly with esomeprazole magnesium delayed-release capsules.
- Take a missed dose as soon as possible. If it is almost time for the next dose, skip the missed dose and take the next dose at the regular scheduled time. Do not take 2 doses at the same time.
Esomeprazole Magnesium Delayed-Release Capsules
Administer esomeprazole magnesium delayed-release capsules orally or via a nasogastric tube, as described below.
Oral Administration
- Swallow esomeprazole magnesium delayed-release capsules whole; do not chew or crush the capsules.
- For patients who have difficulty swallowing capsules, esomeprazole magnesium delayed-release capsules can be opened, and the contents sprinkled on applesauce. Use with other foods has not been evaluated and is not recommended.
1. Add one tablespoon of applesauce to an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
2. Open the esomeprazole magnesium delayed-release capsule and carefully empty the granules inside the capsule onto the applesauce.
3. Mix the granules with the applesauce.
4. Administer the mixture immediately. Do not chew or crush the granules
5. Discard any remaining mixture. Do not store the mixture for future use.
Administration via Nasogastric Tube
1. Open the esomeprazole magnesium delayed-release capsule and empty the granules into a 60 mL catheter-tipped syringe.
2. Mix the granules with 50 mL of water.
3. Replace the plunger and shake the catheter-tipped syringe vigorously for 15 seconds.
4. Hold the catheter-tipped syringe with the tip up and check for any granules remaining in the tip.
5. Attach the catheter-tipped syringe to a nasogastric tube and deliver the contents of the syringe through the nasogastric tube into the stomach.
6. After administering the granules, flush the nasogastric tube with additional water.
7. Use the mixture immediately after preparation. Do not administer the granules if they have dissolved or disintegrated.
-
3 DOSAGE FORMS AND STRENGTHS
- Esomeprazole magnesium delayed-release capsules USP, 20 mg are white opaque size '4' hard gelatin capsule imprinted with “H” on cap and 'E2' on body filled with off white to pale yellow pellets.
- Esomeprazole magnesium delayed-release capsules USP, 40 mg are white opaque size '3' hard gelatin capsule imprinted with “H” on cap and 'E3' on body filled with off white to pale yellow pellets.
-
4 CONTRAINDICATIONS
- Esomeprazole magnesium delayed-release capsules are contraindicated in patients with known hypersensitivity to substituted benzimidazoles or to any component of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute tubulointerstitial nephritis, and urticaria [see Warnings and Precautions ( 5.2) , Adverse Reactions ( 6.2) ].
- For information about contraindications of amoxicillin and clarithromycin, indicated in combination with esomeprazole magnesium delayed-release capsules for H. pylori eradication to reduce the risk of duodenal ulcer recurrence, refer to the Contraindications section of the respective prescribing information.
- Proton pump inhibitors (PPIs), including esomeprazole magnesium, are contraindicated in patients receiving rilpivirine-containing products [see Drug Interactions ( 7) ].
-
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
In adults, symptomatic response to therapy with esomeprazole magnesium does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a PPI. In older patients, also consider an endoscopy.
5.2 Acute Tubulointerstitial Nephritis
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs and may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions to non-specific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia). Discontinue esomeprazole magnesium and evaluate patients with suspected acute TIN [see Contraindications ( 4) ].
5.3 Clostridium difficile-Associated Diarrhea
Published observational studies suggest that PPI therapy like esomeprazole magnesium may be associated with an increased risk of Clostridium difficile-associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions ( 6.2)].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents. For more information specific to antibacterial agents (clarithromycin and amoxicillin) indicated for use in combination with esomeprazole magnesium, refer to Warnings and Precautions section of the corresponding prescribing information.5.4 Bone Fracture
Several published observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see Dosage and Administration ( 2) and Adverse Reactions ( 6.2) ].
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in association with the use of PPIs [see Adverse Reactions ( 6.2) ]. Discontinue esomeprazole magnesium at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including esomeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment primarily in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving esomeprazole magnesium, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serological testing (e.g., ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.
5.7 Interaction with Clopidogrel
Avoid concomitant use of esomeprazole magnesium with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as esomeprazole, that inhibit CYP2C19 activity. Concomitant use of clopidogrel with 40 mg esomeprazole reduces the pharmacological activity of clopidogrel. When using esomeprazole magnesium consider alternative anti-platelet therapy [see Drug Interactions ( 7) ].
5.8 Cyanocobalamin (Vitamin B-12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B-12) caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed.
5.9 Hypomagnesemia and Mineral Metabolism
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures.
Hypomagnesemia may lead to hypocalcemia and/or hypokalemia and may exacerbate underlying hypocalcemia in at-risk patients. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions ( 6.2) ].
Consider monitoring magnesium and calcium levels prior to initiation of esomeprazole magnesium and periodically while on treatment in patients with a preexisting risk of hypocalcemia (e.g., hypoparathyroidism). Supplement with magnesium and/or calcium, as necessary. If hypocalcemia is refractory to treatment, consider discontinuing the PPI.
5.10 Interaction with St. John’s Wort or Rifampin
Drugs which induce CYP2C19 or CYP3A4 (such as St. John’s Wort or rifampin) can substantially decrease esomeprazole concentrations [see Drug Interactions ( 7) ]. Avoid concomitant use of esomeprazole magnesium with St. John’s Wort or rifampin.
5.11 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Healthcare providers should temporarily stop esomeprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Clinical Pharmacology ( 12.2) ].
5.12 Interaction with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions ( 7) ].
5.13 Fundic Gland Polyps
PPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Acute Tubulointerstitial Nephritis [see Warnings and Precautions ( 5.2)]
- Clostridium difficile-Associated Diarrhea [see Warnings and Precautions ( 5.3)]
- Bone Fracture [see Warnings and Precautions ( 5.4)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions ( 5.5)]
- Cutaneous and Systemic Lupus Erythematosus [see Warnings and Precautions ( 5.6)]
- Cyanocobalamin (Vitamin B-12) Deficiency [see Warnings and Precautions ( 5.8)]
- Hypomagnesemia and Mineral Metabolism [see Warnings and Precautions ( 5.9)]
- Fundic Gland Polyps [see Warnings and Precautions ( 5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The safety of esomeprazole magnesium delayed-release capsules was evaluated in over 15,000 patients (aged 18 to 84 years) in clinical trials worldwide including over 8,500 patients in the United States and over 6,500 patients in Europe and Canada. Over 2,900 patients were treated in long-term studies for up to 6 to 12 months.
The safety in the treatment of healing of EE in adults was assessed in four randomized comparative clinical trials, which included 1,240 patients who received esomeprazole magnesium delayed-release capsules 20 mg once daily, 2,434 patients on esomeprazole magnesium delayed-release capsules 40 mg once daily, and 3,008 patients on omeprazole 20 mg once daily. The most frequently occurring adverse reactions (at least 1%) in all three groups were headache (5.5%, 5%, and 3.8%, respectively) and diarrhea (no difference among the three groups). Nausea, flatulence, abdominal pain, constipation, and dry mouth occurred at similar rates among patients taking esomeprazole magnesium or omeprazole.
Less common adverse reactions with an incidence of less than 1% are listed below by body system:
Body as a Whole: abdomen enlarged, allergic reaction, asthenia, back pain, chest pain, substernal chest pain, facial edema, peripheral edema, hot flushes, fatigue, fever, flu-like disorder, generalized edema, leg edema, malaise, pain, rigors;
Cardiovascular: flushing, hypertension, tachycardia;
Endocrine: goiter;
Gastrointestinal: bowel irregularity, constipation aggravated, dyspepsia, dysphagia, dysplasia GI, epigastric pain, eructation, esophageal disorder, frequent stools, gastroenteritis, GI hemorrhage, GI symptoms not otherwise specified, hiccup, melena, mouth disorder, pharynx disorder, rectal disorder, serum gastrin increased, tongue disorder, tongue edema, ulcerative stomatitis, vomiting;
Hearing: earache, tinnitus;
Hematologic: anemia, anemia hypochromic, cervical lymphadenopathy, epistaxis, leukocytosis, leukopenia, thrombocytopenia;
Hepatic: bilirubinemia, hepatic function abnormal, SGOT increased, SGPT increased;
Metabolic/Nutritional: glycosuria, hyperuricemia, hyponatremia, increased alkaline phosphatase, thirst, vitamin B12 deficiency, weight increase, weight decrease;
Musculoskeletal: arthralgia, arthritis aggravated, arthropathy, cramps, fibromyalgia syndrome, hernia, polymyalgia rheumatica;
Nervous System/Psychiatric: anorexia, apathy, appetite increased, confusion, depression aggravated, dizziness, hypertonia, nervousness, hypoesthesia, impotence, insomnia, migraine, migraine aggravated, paresthesia, sleep disorder, somnolence, tremor, vertigo, visual field defect;
Reproductive: dysmenorrhea, menstrual disorder, vaginitis;
Respiratory: asthma aggravated, coughing, dyspnea, larynx edema, pharyngitis, rhinitis, sinusitis;
Skin and Appendages: acne, angioedema, dermatitis, pruritus, pruritus ani, rash, rash erythematous, rash maculo-papular, skin inflammation, sweating increased, urticaria;
Special Senses: otitis media, parosmia, taste loss, taste perversion;
Urogenital: abnormal urine, albuminuria, cystitis, dysuria, fungal infection, hematuria, micturition frequency, moniliasis, genital moniliasis, polyuria;
Visual: conjunctivitis, vision abnormal.
The following potentially clinically significant laboratory changes in clinical trials, irrespective of relationship to esomeprazole magnesium, were reported in 1% or less of patients: increased creatinine, uric acid, total bilirubin, alkaline phosphatase, ALT, AST, hemoglobin, white blood cell count, platelets, serum gastrin, potassium, sodium, thyroxine and thyroid stimulating hormone [see Clinical Pharmacology ( 12.2) ]. Decreases were seen in hemoglobin, white blood cell count, platelets, potassium, sodium, and thyroxine.
Endoscopic findings that were reported as adverse reactions include: duodenitis, esophagitis, esophageal stricture, esophageal ulceration, esophageal varices, gastric ulcer, gastritis, hernia, benign polyps or nodules, Barrett’s esophagus, and mucosal discoloration.
The incidence of adverse reactions during 6-month trials for the maintenance of healing of EE with esomeprazole magnesium delayed-release capsules 20 mg once daily was similar to placebo. There were no differences in types of adverse reactions seen during maintenance treatment up to 12 months compared to short-term treatment.
Two placebo-controlled studies were conducted in 710 adult patients for the treatment of symptomatic GERD. The most common adverse reactions that were reported were: diarrhea (4%), headache (4%), and abdominal pain (4%).
Combination Treatment with Esomeprazole Magnesium, Amoxicillin and Clarithromycin
In clinical trials of H. pylori eradication of to reduce duodenal ulcer recurrence, no additional adverse reactions specific to the combination of esomeprazole magnesium, amoxicillin and clarithromycin were observed and were similar to those observed with esomeprazole magnesium, amoxicillin, or clarithromycin alone. The most frequently reported adverse reactions for patients who received esomeprazole magnesium delayed-release capsules, amoxicillin and clarithromycin for 10 days were diarrhea (9%), taste perversion (4%), and abdominal pain (4%). No adverse reactions were observed at higher rates with esomeprazole magnesium, amoxicillin and clarithromycin than were observed with esomeprazole magnesium alone.
In clinical trials using of esomeprazole magnesium, amoxicillin and clarithromycin, no additional increased laboratory abnormalities particular to these drug combinations were observed.
For more information on adverse reactions and laboratory changes with amoxicillin or clarithromycin, refer to Adverse Reactions section of the respective prescribing information.
Pediatrics
1 Year to 17 Years of Age
The safety of esomeprazole magnesium delayed-release capsules was evaluated in 316 pediatric and adolescent patients aged 1 year to 17 years in four clinical trials for the treatment of symptomatic GERD [see Clinical Studies ( 14.3) ]. In 109 pediatric patients aged 1 year to 11 years, the most frequently reported (at least 1%) treatment-related adverse reactions in these patients were diarrhea (3%), headache (2%) and somnolence (2%). In 149 pediatric patients aged 12 years to 17 years the most frequently reported adverse reactions (at least 2%) were headache (8%), abdominal pain (3%), diarrhea (2%), and nausea (2%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of esomeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system:
Blood and Lymphatic: agranulocytosis, pancytopenia;
Eye: blurred vision;
Gastrointestinal: pancreatitis; stomatitis; microscopic colitis; fundic gland polyps;
Hepatobiliary: hepatic failure, hepatitis with or without jaundice;
Immune System: anaphylactic reaction/shock; systemic lupus erythematosus;
Infections and Infestations: GI candidiasis; Clostridium difficile-associated diarrhea;
Metabolism and nutritional disorders: hypomagnesemia (may lead to hypocalcemia and/or hypokalemia) [see Warnings and Precautions (5.9)];
Musculoskeletal and Connective Tissue: muscular weakness, myalgia, bone fracture;
Nervous System: hepatic encephalopathy, taste disturbance;
Psychiatric: aggression, agitation, depression, hallucination;
Renal and Urinary: interstitial nephritis;
Reproductive System and Breast: gynecomastia, erectile dysfunction;
Respiratory, Thoracic, and Mediastinal: bronchospasm;
Skin and Subcutaneous Tissue: alopecia, erythema multiforme, hyperhidrosis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP), cutaneous lupus erythematosus.
Adverse reactions associated with omeprazole may also be expected to occur with esomeprazole. See the full prescribing information for omeprazole for complete safety information.
-
7 DRUG INTERACTIONS
Tables 3 and 4 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with esomeprazole and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Table 3: Clinically Relevant Interactions Affecting Drugs Co-Administered with Esomeprazole and Interaction with Diagnostics
Antiretrovirals
Clinical Impact:
The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir, and nelfinavir) when used concomitantly with esomeprazole may reduce antiviral effect and promote the development of drug resistance [see Clinical Pharmacology (12.3)].
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with esomeprazole may increase toxicity [see Clinical Pharmacology (12.3)].
- There are other antiretroviral drugs which do not result in clinically relevant interactions with esomeprazole.
Intervention:
Rilpivirine-containing products: Concomitant use with esomeprazole magnesium is contraindicated [see Contraindications (4)].
Atazanavir: See prescribing information for atazanavir for dosing information.
Nelfinavir: Avoid concomitant use with esomeprazole magnesium. See prescribing information for nelfinavir.
Saquinavir: See the prescribing information for saquinavir for monitoring of potential saquinavir-related toxicities.
Other antiretrovirals: See prescribing information for specific antiretroviral drugs
Warfarin
Clinical Impact:
Increased INR and prothrombin time in patients receiving PPIs, including esomeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Intervention:
Monitor INR and prothrombin time and adjust the dose of warfarin, if needed, to maintain the target INR range.
Methotrexate
Clinical Impact:
Concomitant use of esomeprazole with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted [see Warnings and Precautions (5.12)].
Intervention:
A temporary withdrawal of esomeprazole magnesium may be considered in some patients receiving high-dose methotrexate.
2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol)
Clopidogrel
Clinical Impact:
Concomitant use of esomeprazole 40 mg resulted in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition [see Clinical Pharmacology (12.3)].
There are no adequate combination studies of a lower dose of esomeprazole or a higher dose of clopidogrel in comparison with the approved dose of clopidogrel.
Intervention:
Avoid concomitant use with esomeprazole magnesium delayed-release capsules Consider use of alternative anti-platelet therapy [see Warnings and Precautions (5.7)].
Citalopram
Clinical Impact:
Increased exposure of citalopram leading to an increased risk of QT prolongation [see Clinical Pharmacology (12.3)].
Intervention:
Limit the dose of citalopram to a maximum of 20 mg per day. See prescribing information for citalopram.
Cilostazol
Clinical Impact:
Increased exposure of cilostazol and one of its active metabolites (3,4-dihydro-cilostazol) [see Clinical Pharmacology (12.3)].
Intervention:
Consider reducing the dose of cilostazol to 50 mg twice daily. See prescribing information for cilostazol.
Digoxin
Clinical Impact:
Potential for increased exposure of digoxin [see Clinical Pharmacology (12.3)].
Intervention:
Monitor digoxin concentrations and adjust the dose, if needed, to maintain therapeutic drug concentrations. See prescribing information for digoxin.
Combination Therapy with Clarithromycin and Amoxicillin
Clinical Impact:
Concomitant administration of clarithromycin with other drugs can lead to serious adverse reactions, including potentially fatal arrhythmias, and are contraindicated.
Amoxicillin also has drug interactions.
Intervention:
See Contraindications, Warnings and Precautionsin prescribing information for clarithromycin.
See Drug Interactions in prescribing information for amoxicillin.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)
Clinical Impact:
Esomeprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity
Intervention:
Mycophenolate mofetil (MMF): Co-administration of omeprazole, of which esomeprazole is an enantiomer, in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving esomeprazole magnesium and MMF. Use esomeprazole magnesium with caution in transplant patients receiving MMF [see Clinical Pharmacology (12.3)].
See the prescribing information for other drugs dependent on gastric pH for absorption.
Tacrolimus
Clinical Impact:
Potentially increased exposure of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
Intervention:
Monitor tacrolimus whole blood concentrations and consider reducing the dose, if needed, to maintain therapeutic drug concentrations. See prescribing information for tacrolimus.
Interactions with Investigations of Neuroendocrine Tumors
Clinical Impact:
Serum chromogranin A (CgA) levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.11), Clinical Pharmacology (12.2)].
Intervention:
Discontinue esomeprazole magnesium at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Interaction with Secretin Stimulation Test
Clinical Impact:
Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma.
Intervention:
Discontinue esomeprazole magnesium 4 weeks prior to testing [see Clinical Pharmacology (12.2)]
False Positive Urine Tests for THC
Clinical Impact:
There have been reports of false positive urine screening test for tetrahydrocannabinol (THC) in patients receiving PPIs.
Intervention:
An alternative confirmatory method should be considered to verify positive results.
Table 4: Clinically Relevant Interactions Affecting Esomeprazole When Co-Administered with Other Drugs
CYP2C19 or CYP3A4 Inducers
Clinical Impact:
Decreased exposure of esomeprazole when used concomitantly with strong inducers [see Clinical Pharmacology (12.3)].
Intervention:
St. John’s Wort, rifampin:Avoid concomitant use with [see Warnings and Precautions (5.10)].
Ritonavir-containing products:see prescribing information for specific drugs
Voriconazole
Clinical Impact:
Increased exposure of esomeprazole [see Clinical Pharmacology (12.3)].
Intervention:
Dose adjustment of esomeprazole magnesium is not normally required. However, in patients with Zollinger-Ellison syndrome, who may require higher doses, dosage adjustment may be considered.
See prescribing information for voriconazole.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with esomeprazole in pregnant women. Esomeprazole is the S-isomer of omeprazole. Available epidemiologic data fail to demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use (see Data). Reproduction studies in rats and rabbits resulted in dose-dependent embryo-lethality at omeprazole doses that were approximately 3.4 to 34 times an oral human dose of 40 mg (based on a body surface area for a 60 kg person).
Teratogenicity was not observed in animal reproduction studies with administration of oral esomeprazole magnesium in rats and rabbits with doses about 68 times and 42 times, respectively, an oral human dose of 40 mg (based on a body surface area basis for a 60 kg person). Changes in bone morphology were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 34 times an oral human dose of 40 mg. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age ( see Data).
The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Esomeprazole is the S-isomer of omeprazole. Four epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, from 1995 to 1999, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed in utero to omeprazole that had any malformation, low birth weight, low Apgar score, or hospitalization was similar to the number observed in this population. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole-exposed infants than the expected number in this population.
A population-based retrospective cohort study covering all live births in Denmark from 1996 to 2009, reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837,317 live births whose mothers did not use any proton pump inhibitor. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any proton pump inhibitor during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2-blocker, or were unexposed was 3.6%, 5.5%, and 4.1% respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% with first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Animal Data
Omeprazole
Reproductive studies conducted with omeprazole in rats at oral doses up to 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at doses up to 69.1 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) during organogenesis did not disclose any evidence for a teratogenic potential of omeprazole. In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis) administered during organogenesis produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis), administered prior to mating through the lactation period.
Esomeprazole
No effects on embryo-fetal development were observed in reproduction studies with esomeprazole magnesium in rats at oral doses up to 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) or in rabbits at oral doses up to 86 mg/kg/day (about 41 times an oral human dose of 40 mg on a body surface area basis) administered during organogenesis.
A pre- and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development was performed with esomeprazole magnesium at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg on a body surface area basis). Neonatal/early postnatal (birth to weaning) survival was decreased at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis). Body weight and body weight gain were reduced and neurobehavioral or general developmental delays in the immediate post-weaning timeframe were evident at doses equal to or greater than 69 mg/kg/day (about 17 times an oral human dose of 40 mg on a body surface area basis). In addition, decreased femur length, width and thickness of cortical bone, decreased thickness of the tibial growth plate and minimal to mild bone marrow hypocellularity were noted at doses equal to or greater than 14 mg/kg/day (about 3.4 times an oral human dose of 40 mg on a body surface area basis). Physeal dysplasia in the femur was observed in offspring of rats treated with oral doses of esomeprazole magnesium at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis).
Effects on maternal bone were observed in pregnant and lactating rats in a pre- and postnatal toxicity study when esomeprazole magnesium was administered at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg on a body surface area basis). When rats were dosed from gestational day 7 through weaning on postnatal day 21, a statistically significant decrease in maternal femur weight of up to 14% (as compared to placebo treatment) was observed at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis).
A pre- and postnatal development study in rats with esomeprazole strontium (using equimolar doses compared to esomeprazole magnesium study) produced similar results in dams and pups as described above.
A follow up developmental toxicity study in rats with further time points to evaluate pup bone development from postnatal day 2 to adulthood was performed with esomeprazole magnesium at oral doses of 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) where esomeprazole administration was from either gestational day 7 or gestational day 16 until parturition. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age.
8.2 Lactation
Risk Summary
Esomeprazole is the S-isomer of omeprazole and limited data suggest that omeprazole may be present in human milk. There are no clinical data on the effects of esomeprazole on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for esomeprazole magnesium delayed-release capsules and any potential adverse effects on the breastfed infant from esomeprazole magnesium delayed-release capsules or from the underlying maternal condition.
8.4 Pediatric Use
Healing of EE
Pediatric Patients 1 Year to 17 Years of Age
The safety and effectiveness of esomeprazole magnesium delayed-release capsules have been established in pediatric patients 12 years to 17 years for short-term treatment (4 to 8 weeks) for healing of EE. Use of esomeprazole magnesium delayed-release capsules for this indication is supported by evidence from adequate and well-controlled studies in adults with additional safety and pharmacokinetic data in pediatric patients 1 year to 17 years of age. The safety profile in pediatric patients 1 year to 17 years of age was similar to adults [see Adverse Reactions ( 6.1) , Clinical Pharmacology ( 12.3) , Clinical Studies ( 14.4) ].
Symptomatic GERD
Pediatric Patients 1 Year to 17 Years of Age
The safety and effectiveness of esomeprazole magnesium delayed-release capsules have been established in pediatric patients 12 years to 17 years of age for the short-term treatment (4 weeks) of heartburn and other symptoms associated with GERD. Use of esomeprazole magnesium for this indication is supported by evidence from adequate and well-controlled studies in adults with additional safety and pharmacokinetic data in pediatric patients 1 year to 17 years of age. The safety profile in pediatric patients 1 year to 17 years of age was similar to adults [see Adverse Reactions ( 6.1) , Clinical Pharmacology ( 12.3) , Clinical Studies ( 14.4) ].
The safety and effectiveness of esomeprazole magnesium for the treatment of symptomatic GERD in pediatric patients less than 1 year of age have not been established.
Other Conditions
The safety and effectiveness of esomeprazole magnesium for the risk reduction of NSAID-associated gastric ulcer, H. pylorieradication to reduce the risk of duodenal ulcer recurrence and treatment of pathological hypersecretory conditions have not been established in pediatric patients.
Juvenile Animal Toxicity Studies
In a juvenile rat toxicity study, esomeprazole was administered with both magnesium and strontium salts at oral doses about 34 to 68 times a daily human dose of 40 mg based on body surface area. Increases in death were seen at the high dose, and at all doses of esomeprazole, there were decreases in body weight, body weight gain, femur weight and femur length, and decreases in overall growth [see Nonclinical Toxicology (13.2)].8.5 Geriatric Use
Of the total number of patients who received esomeprazole magnesium in clinical trials, 1459 were 65 to 74 years of age and 354 patients were 75 years of age and older.
No overall differences in safety and efficacy were observed between the elderly and younger individuals, and other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
In patients with severe hepatic impairment (Child-Pugh Class C) exposure to esomeprazole substantially increased compared to healthy subjects. Dosage modification of esomeprazole magnesium delayed-release capsules is recommended for patients with severe hepatic impairment for the healing of EE, risk reduction of NSAID-associated gastric ulcer, H. pylorieradication to reduce the risk of duodenal ulcer recurrence, and pathological hypersecretory conditions including Zollinger-Ellison Syndrome [see Dosage and Administration ( 2.1), Clinical Pharmacology ( 12.3)].
In patients with mild to moderate liver impairment (Child-Pugh Classes A and B), no dosage adjustment is necessary. -
10 OVERDOSAGE
Manifestations in patients exposed to omeprazole, the racemic mixture, at doses up to 2,400 mg (120 times the usual recommended clinical dose) include confusion, drowsiness, blurred vision, tachycardia, nausea, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen at recommended dosages. See the full prescribing information for omeprazole for complete safety information. No specific antidote for esomeprazole is known. Since esomeprazole is extensively protein bound, it is not expected to be removed by dialysis. In the event of overdosage, treatment should be symptomatic and supportive.
If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
-
11 DESCRIPTION
The active ingredient in the esomeprazole magnesium delayed-release capsules, USP for oral administration is 1H-Benzimidazole, 5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl- 2-pyridinyl]methyl] Sulfinyl], Magnesium Salt (2:1) Trihydrate. Esomeprazole is S-enantiomer of omeprazole. (Initial U.S. approval of esomeprazole magnesium: 2001). Its molecular formula is C 34H 36MgN 6O 6S 2.3H 2O with molecular weight of 767.17 as a trihydrate. The structural formula is:
Figure 1
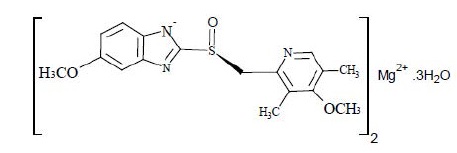
The magnesium salt is a white to slightly colored powder. It contains 3 moles of water. Slightly soluble in methanol, insoluble in water and in n-Heptane. The stability of esomeprazole magnesium is a function of pH; it rapidly degrades in acidic media, but it has acceptable stability under alkaline conditions.
Esomeprazole magnesium is supplied in delayed-release capsules. Each delayed-release capsule contains 20 mg, or 40 mg of esomeprazole (equivalent to 22.25 mg esomeprazole magnesium trihydrate, USP) or 40 mg of esomeprazole (equivalent to 44.5 mg esomeprazole magnesium trihydrate, USP) in the form of enteric-coated granules with the following inactive ingredients: glyceryl monostearate, hydroxy propyl cellulose, hypromellose, magnesium stearate, methacrylic acid ethyl acrylate copolymer, polysorbate 80, simethicone, sugar spheres (contains sucrose and starch), talc and triethyl citrate. The capsule shells have the following inactive ingredients: gelatin, titanium dioxide and sodium lauryl sulfate.
The printing ink contains shellac, propylene glycol, strong ammonia solution, black iron oxide and potassium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Esomeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that suppress gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Esomeprazole is protonated and converted in the acidic compartment of the parietal cell forming the active inhibitor, the achiral sulphenamide. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, esomeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus.
12.2 Pharmacodynamics
Antisecretory Activity
Adults
The effect of esomeprazole on intragastric pH was determined in adult patients with symptomatic GERD in two separate studies. In the first study of 36 patients, esomeprazole magnesium 40 mg and 20 mg delayed-release capsules were administered once daily over 5 days as shown in Table 5:
Table 5: Effect of Esomeprazole on Intragastric pH on Day 5 (N=36) Following Once Daily Dosing of Esomeprazole Magnesium Delayed-Release Capsules in Adult Patients with Symptomatic GERD
Parameter
Esomeprazole Magnesium Delayed-Release Capsules
40 mg once daily
20 mg once daily
% Time Gastric pH >4 1 (Hours)
70% 2
(16.8 h)
53%
(12.7 h)
Coefficient of variation
26%
37%
Median 24 Hour pH
4.9 2
4.1
Coefficient of variation
16%
27%
1.Gastric pH was measured over a 24-hour period
2.p< 0.01 esomeprazole magnesium 40 mg vs. Esomeprazole magnesium 20 mg
In a second study, the effect on intragastric pH of esomeprazole magnesium 40 mg delayed-release capsules administered once daily over a five-day period was similar to the first study, (% time with pH > 4 was 68% or 16.3 hours).
Serum Gastrin Effects
The effect of esomeprazole on serum gastrin concentrations was evaluated in approximately 2,700 patients in clinical trials of oral esomeprazole for up to 8 weeks and in over 1,300 patients for up to 12 months. The mean fasting gastrin level increased in a dose-related manner. The increase in serum gastrin concentrations reached a plateau within two to three months of therapy and returned to baseline levels within four weeks after discontinuation of therapy.
Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum Chromogranin A (CgA) levels. The increased CgA levels may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.11)]
Enterochromaffin-like (ECL) Cell Effects
Human gastric biopsy specimens have been obtained from more than 3,000 patients (both pediatrics and adults) treated with omeprazole in long-term clinical trials. The incidence of ECL cell hyperplasia in these studies increased with time; however, no case of ECL cell carcinoids, dysplasia, or neoplasia has been found in these patients [see Nonclinical Toxicology (13.1)]
In over 1,000 patients treated with oral esomeprazole (10 mg, 20 mg or 40 mg/day) for up to 12 months, the prevalence of ECL cell hyperplasia increased with time and dose. No patient developed ECL cell carcinoids, dysplasia, or neoplasia in the gastric mucosa.
Endocrine Effects
Esomeprazole had no effect on thyroid function in adults when given esomeprazole magnesium 20 mg or 40 mg delayed-release capsules once daily for 4 weeks. Other effects of esomeprazole on the endocrine system were assessed in studies of omeprazole. Oral doses of omeprazole 30 mg or 40 mg once daily for 2 to 4 weeks had no effect on carbohydrate metabolism, circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin, or secretin.
12.3 Pharmacokinetics
Absorption
Esomeprazole magnesium delayed-release capsules and esomeprazole magnesium delayed-release oral suspension showed similar bioavailability after a single dose (40 mg) administration in 94 healthy male and female subjects under fasting conditions. After oral administration, peak plasma levels (C max) of esomeprazole occur at approximately 1.5 hours (T max). The C max increases proportionally when the dose is increased, and there is a three-fold increase in the area under the plasma concentration-time curve (AUC) from 20 to 40 mg. At repeated once-daily dosing with 40 mg, the systemic bioavailability is approximately 90% compared to 64% after a single dose of 40 mg. The mean exposure (AUC) to esomeprazole increases from 4.32 micromol*hr/L on Day 1 to 11.2 micromol*hr/L on Day 5 after 40 mg once daily dosing.
The AUC after administration of a single 40 mg dose of esomeprazole magnesium delayed-release capsules is decreased by 43% to 53% after food intake compared to fasting conditions [see Dosage and Administration ( 2.3)] . The pharmacokinetics of esomeprazole in adult patients with symptomatic GERD following repeated once daily administration of 20 mg and 40 mg esomeprazole magnesium delayed-release capsules over a period of five days are shown in Table 6:
Table 6: Geometric Mean (95% CI) Pharmacokinetic Parameters of Esomeprazole on Day 5 Following Once Daily Dosing of Esomeprazole Magnesium Delayed-Release Capsules in Adult Patients with Symptomatic GERD
Esomeprazole Magnesium Delayed-Release Capsules
Parameter 1(CV)
40 mg once daily
(n=36)
20 mg once daily
(n=36)
AUC (micromol·h/L)
12.6 (42%)
4.2 (59%)
C max(micromol/L)
4.7 (37%)
2.1 (45%)
T max(hours)
1.6
1.6
t 1/2(hours)
1.5
1.2
1.Values represent the geometric mean, except the T max, which is the arithmetic mean; CV = Coefficient of variation
Esomeprazole is a time-dependent inhibitor of CYP2C19, resulting in autoinhibition and nonlinear pharmacokinetics. The systemic exposure increases in a more than dose proportional manner after multiple oral doses of esomeprazole. Compared to the first dose, the systemic exposure (C max and AUC 0-24h) at steady state following once a day dosing increased by 43% and 90%, respectively, compared to after the first dose for the 20 mg dose and increased by 95% and 159%, respectively, for the 40 mg dose.
Distribution
Esomeprazole is 97% bound to plasma proteins. Plasma protein binding is constant over the concentration range of 2 to 20 micromol/L. The apparent volume of distribution at steady state in healthy subjects is approximately 16 L.
Elimination
Metabolism
Esomeprazole is extensively metabolized in the liver by the cytochrome P450 (CYP) enzyme system. The metabolites of esomeprazole lack antisecretory activity. The major part of esomeprazole’s metabolism is dependent upon the CYP2C19 isoenzyme, which forms the hydroxy and desmethyl metabolites. The remaining amount is dependent on CYP3A4 which forms the sulphone metabolite.
Excretion
The plasma elimination half-life of esomeprazole is approximately 1 to 1.5 hours. Less than 1% of parent drug is excreted in the urine. Approximately 80% of an oral dose of esomeprazole is excreted as inactive metabolites in the urine, and the remainder is found as inactive metabolites in the feces.
Combination Therapy with Amoxicillin and Clarithromycin
Esomeprazole magnesium delayed-release capsules 40 mg once daily was given in combination with amoxicillin 1000 mg twice daily and clarithromycin 500 mg twice daily for 7 days to 17 healthy male and female subjects. The mean steady state AUC and C max of esomeprazole increased by 70% and 18%, respectively during combination therapy compared to treatment with esomeprazole magnesium delayed-release capsules alone. The observed increase in esomeprazole exposure during co-administration with amoxicillin and clarithromycin is not expected to be clinically relevant.
The pharmacokinetic parameters for amoxicillin and clarithromycin were similar during combination therapy and administration of each drug alone. However, the mean AUC and C max for 14-hydroxyclarithromycin increased by 19% and 22%, respectively, during combination therapy compared to treatment with clarithromycin alone. This increase in exposure to 14-hydroxyclarithromycin is not considered to be clinically relevant.
Specific Populations
Geriatric Patients
The AUC and C max values of esomeprazole were slightly higher (25% and 18%, respectively) in the elderly as compared to younger subjects at steady state. This increase in exposure is not considered clinically relevant.
Pediatric Patients
1 Month to 11 Months of Age
The pharmacokinetic parameters following repeated dose administration of esomeprazole magnesium 1 mg/kg once daily for 7 to 8 days in 1 month to 11-month-old infants with GERD are summarized in Table 7.
Table 7: Summary of Esomeprazole Pharmacokinetic Parameters Following Once Daily Dosing of Oral Esomeprazole Magnesium for 7 to 8 Days in 1 Month to 1 Year Old Infants with GERD
Parameter
Esomeprazole Magnesium 1 mg/kg Orally Once Daily
AUC (micromol·h/L) (n=7) 1
3.51
C ss,max(micromol/L) (n=15) 1
0.87
t ½ (h) (n=8 )1
0.93
t max (h) (n=15) 2
3
1.Geometric mean
2.Median
Subsequent pharmacokinetic simulation analyses showed that for pediatric patients 1 month to 11 months of age, a dosage regimen of 2.5 mg once daily (body weight 3 to 5 kg), 5 mg once daily (body weight more than 5 to 7.5 kg) and 10 mg once daily for (body weight more than 7.5 to 12 kg) would achieve comparable steady-state plasma exposures (AUC) to that observed with 10 mg once daily in patients 1 year to 11 year of age and 20 mg once daily in patients 12 years to 18 years of age, as well as adults.
Apparent clearance (CL/F) increases with age in pediatric patients with GERD from 1 month to 2 years of age.
1 Year to 11 Years of Age
The pharmacokinetics of esomeprazole were studied in pediatric patients with GERD aged 1 year to 11 years. Following once daily dosing with esomeprazole magnesium delayed-release oral suspension for 5 days, the total exposure (AUC) for the 10 mg dosage in patients aged 6 years to 11 years was similar to that seen with the 20 mg dosage in adults and adolescents aged 12 years to 17 years. The total exposure for the 10 mg dosage in patients aged 1 year to 5 years was approximately 30% higher than the 10 mg dosage in patients aged 6 years to 11 years. The total exposure for the 20 mg dosage in patients aged 6 years to 11 years was higher than that observed with the 20 mg dosage in patients aged 12 years to 17 years and adults, but lower than that observed with the 40 mg dosage in 12 to 17 year-olds and adults. See Table 8.
Table 8: Summary of Esomeprazole Pharmacokinetic Parameters Following Once Daily Dosing of Esomeprazole magnesium for Delayed-Release Oral Suspension for 5 Days in 1 Year to 11 Year Old Patients with GERD
Esomeprazole magnesium For Delayed-Release Oral Suspension
1 Year to 5 Years
6 Years to 11 Years
Parameter
10 mg once daily
(N = 8)
10 mg once daily
(N = 7)
20 mg once daily
(N = 6)
AUC (micromol*h/L) 1
4.83
3.7
6.28
C max (micromol/L) 1
2.98
1.77
3.73
t max (h) 2
1.44
1.79
1.75
t ½λz (h) 1
0.74
0.88
0.73
Cl/F (L/h) 1
5.99
7.84
9.22
1Geometric mean
2Arithmetic mean
12 Years to 17 Years of Age
The pharmacokinetics of esomeprazole magnesium were studied in 28 adolescent patients with GERD aged 12 to 17 years inclusive, in a single center study. Patients were randomized to receive esomeprazole magnesium 20 mg or 40 mg once daily for 8 days. Mean Cmax and AUC values of esomeprazole were not affected by body weight or age; and more than dose-proportional increases in mean Cmax and AUC values were observed between the two dose groups in the study. Overall, esomeprazole magnesium pharmacokinetics in adolescent patients aged 12 to 17 years were similar to those observed in adult patients with symptomatic GERD. See Table 9.
Table 9: Comparison of Esomeprazole Pharmacokinetic Parameters Following Once Daily Dosing of Esomeprazole Magnesium Delayed-Release Capsules in Pediatric Patients 12 Years to 17 Years with GERD and Adults with Symptomatic GERD 1
Parameter
Esomeprazole Magnesium Delayed-Release Capsules
12 Years to 17 Years (N=28)
Adults (N=36)
20 mg once daily
for 8 days
40 mg once daily
for 8 days
20 mg once daily
for 5 days
40 mg once daily
for 5 days
AUC (micromol .h/L)
3.65
13.86
4.2
12.6
C max(micromol/L)
1.45
5.13
2.1
4.7
t max(h)
2
1.75
1.6
1.6
t½λz(h)
0.82
1.22
1.2
1.5
Data presented are geometric means for AUC, C maxand t½λz, and median value for t max.
1Data obtained from two independent studies.
Male and Female Patients
The AUC and C max values of esomeprazole were slightly higher (13%) in females than in males at steady state when dosed orally. This increase in exposure is not considered clinically relevant.
Patients with Renal Impairment
The pharmacokinetics of esomeprazole magnesium delayed-release capsules in patients with renal impairment are not expected to be altered relative to healthy subjects as less than 1% of esomeprazole is excreted unchanged in urine.
Patients with Hepatic Impairment
The steady state pharmacokinetics of esomeprazole obtained after administration of esomeprazole magnesium delayed-release capsules 40 mg orally once daily to patients with mild (Child-Pugh Class A, n=4), moderate (Child-Pugh Class B, n=4), and severe (Child-Pugh Class C, n=4) hepatic impairment were compared to those obtained in 36 male and female GERD patients with normal liver function. In patients with mild and moderate hepatic impairment, the AUCs were within the range that could be expected in patients with normal liver function. In patients with severe hepatic impairment the AUCs were 2 to 3 times higher than in the patients with normal liver function [see Use in Specific Populations ( 8.6)].
Drug Interaction Studies
Effect of Esomeprazole/Omeprazole on Other Drugs
In vitro and in vivo studies have shown that esomeprazole is not likely to inhibit CYPs 1A2, 2A6, 2C9, 2D6, 2E1 and 3A4.
Antiretrovirals
For some antiretroviral drugs, such as rilpivirine, atazanavir and nelfinavir, decreased serum concentrations have been reported when given together with omeprazole [see Drug Interactions ( 7) ].
Rilpivirine:
Following multiple doses of rilpivirine (150 mg, daily) and omeprazole (20 mg, daily), AUC was decreased by 40%, C max by 40%, and Cmin by 33% for rilpivirine [see Contraindications ( 4) ].
Nelfinavir:
Following multiple doses of nelfinavir (1250 mg, twice daily) and omeprazole (40 mg daily), AUC was decreased by 36% and 92%, C max by 37% and 89% and Cmin by 39% and 75% respectively for nelfinavir and M8.
Atazanavir:
Following multiple doses of atazanavir (400 mg, daily) and omeprazole (40 mg, daily, 2 hours before atazanavir), AUC was decreased by 94%, C max by 96%, and C min by 95%.
Saquinavir:
Following multiple dosing of saquinavir/ritonavir (1000/100 mg) twice daily for 15 days with omeprazole 40 mg daily co-administered days 11 to 15. The AUC was increased by 82%, C max by 75%, and C min by 106%. The mechanism behind this interaction is not fully elucidated.
Clopidogrel
In a crossover study, healthy subjects were administered clopidogrel (300 mg loading dose followed by 75 mg per day as the maintenance dosage for 28 days) alone and with esomeprazole (40 mg orally once daily at the same time as clopidogrel) for 29 days. Exposure to the active metabolite of clopidogrel was reduced by 35% to 40% over this time period when clopidogrel and esomeprazole were administered together. Pharmacodynamic parameters were also measured and demonstrated that the change in inhibition of platelet aggregation was related to the change in the exposure to clopidogrel active metabolite [see Warnings and Precautions ( 5.7) and Drug Interactions ( 7) ].
Mycophenolate Mofetil
Administration of omeprazole 20 mg twice daily for 4 days and a single 1000 mg dose of MMF approximately one hour after the last dose of omeprazole to 12 healthy subjects in a cross-over study resulted in a 52% reduction in the C max and 23% reduction in the AUC of MPA [see Drug Interactions ( 7) ].
Cilostazol
Omeprazole acts as an inhibitor of CYP2C19. Omeprazole, given in doses of 40 mg daily for one week to 20 healthy subjects in cross-over study, increased C max and AUC of cilostazol by 18% and 26% respectively. The C max and AUC of one of the active metabolites, 3,4-dihydro-cilostazol, which has 4 to 7 times the activity of cilostazol, were increased by 29% and 69%, respectively. Co-administration of cilostazol with omeprazole is expected to increase concentrations of cilostazol and the above mentioned active metabolite [see Drug Interactions ( 7) ].
Diazepam
Co-administration of esomeprazole 30 mg and diazepam, a CYP2C19 substrate, resulted in a 45% decrease in clearance of diazepam. Increased plasma levels of diazepam were observed 12 hours after dosing and onwards. However, at that time, the plasma levels of diazepam were below the therapeutic interval, and thus this interaction is unlikely to be of clinical relevance.
Digoxin
Concomitant administration of omeprazole 20 mg once daily and digoxin in healthy subjects increased the bioavailability of digoxin by 10% (30% in two subjects) [see Drug Interactions ( 7) ].
Other Drugs
Concomitant administration of esomeprazole and either naproxen (non-selective NSAID) did not identify any clinically relevant changes in the pharmacokinetic profiles of these NSAIDs.
Effect of Other Drugs on Esomeprazole/Omeprazole
St. John’s Wort
In a cross-over study in 12 healthy male subjects, St. John’s Wort (300 mg three times daily for 14 days) significantly decreased the systemic exposure of omeprazole in CYP2C19 poor metabolizers (C max and AUC both decreased by 38%) and extensive metabolizers (C max and AUC decreased by 50% and 44%, respectively) [see Drug Interactions ( 7) ].
Voriconazole
Concomitant administration of omeprazole and voriconazole (a combined inhibitor of CYP2C19 and CYP3A4) resulted in more than doubling of the omeprazole exposure. When voriconazole (400 mg every 12 hours for one day, followed by 200 mg once daily for 6 days) was given with omeprazole (40 mg once daily for 7 days) to healthy subjects, the steady-state C max and AUC 0-24 of omeprazole significantly increased: an average of 2 times (90% CI: 1.8, 2.6) and 4 times (90% CI: 3.3, 4.4), respectively, as compared to when omeprazole was given without voriconazole [see Drug Interactions ( 7) ].
Other Drugs
Co-administration of esomeprazole with oral contraceptives, diazepam, phenytoin, quinidine, naproxen (non-selective NSAID) did not seem to change the pharmacokinetic profile of esomeprazole.12.4 Microbiology
Esomeprazole magnesium, amoxicillin, and clarithromycin triple therapy has been shown to be active against most strains of Helicobacter pylori (H. pylori) in vitro and in clinical infections [see Indications and Usage ( 1) and Clinical Studies ( 14) ].
Helicobacter pylori: Susceptibility testing of H. pylori isolates was performed for amoxicillin and clarithromycin using agar dilution methodology, and minimum inhibitory concentrations (MICs) were determined.
Pretreatment Resistance: Clarithromycin pretreatment resistance rate (MIC ≥ 1 mcg/mL) to H. pylori was 15% (66/445) at baseline in all treatment groups combined. A total of > 99% (394/395) of patients had H. pylori isolates that were considered to be susceptible (MIC ≤ 0.25 mcg/mL) to amoxicillin at baseline. One patient had a baseline H. pylori isolate with an amoxicillin MIC = 0.5 mcg/mL.
Clarithromycin Susceptibility Test Results and Clinical/Bacteriologic Outcomes: The baseline H. pylori clarithromycin susceptibility results and the H. pylori eradication results at the Day 38 visit are shown in Table 10:
Table 10: Clarithromycin Susceptibility Test Results and Clinical/Bacteriological Outcomes 1for Triple Therapy (Esomeprazole Magnesium Delayed-Release Capsules 40 mg once daily, Amoxicillin 1000 mg twice daily and Clarithromycin 500 mg twice daily for 10 days)
Clarithromycin
Pretreatment Results
H. pylorinegative
(Eradicated)
H.pyloripositive
(Not Eradicated)
Post-treatment susceptibility results
S 2
I 2
R 2
No MIC
Susceptible 2
182
162
4
0
2
14
Intermediate 2
1
1
0
0
0
0
Resistant 2
29
13
1
0
13
2
1Includes only patients with pretreatment and post-treatment clarithromycin susceptibility test results
2Susceptible (S) MIC ≤ 0.25 mcg/mL, Intermediate (I) MIC = 0.5 mcg/mL, Resistant (R) MIC ≥ 1.0 mcg/mL
Patients not eradicated of H. pylori following triple therapy with esomeprazole magnesium, amoxicillin and clarithromycin will likely have clarithromycin resistant H. pylori isolates. Therefore, clarithromycin susceptibility testing should be done, when possible. Patients with clarithromycin resistant H. pylori should not be re-treated with a clarithromycin-containing regimen.
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes:
In patients treated with esomeprazole magnesium, amoxicillin and clarithromycin in clinical trials, 83% (176/212) of the patients who had pretreatment amoxicillin susceptible MICs (≤ 0.25 mcg/mL) were eradicated of H. pylori, and 17% (36/212) were not eradicated of H. pylori. Of the 36 patients who were not eradicated of H. pylori, 16 had no post-treatment susceptibility test results and 20 had post-treatment H. pylori isolates with amoxicillin susceptible MICs. Fifteen of the patients who were not eradicated of H. pylori also had post-treatment H. pylori isolates with clarithromycin resistant MICs. There were no patients with H. pylori isolates who developed treatment emergent resistance to amoxicillin.
Susceptibility Test for Helicobacter pylori: For susceptibility testing information about Helicobacter pylori, see Microbiology section in prescribing information for clarithromycin and amoxicillin.
Effects on Gastrointestinal Microbial Ecology: Decreased gastric acidity due to any means, including proton pump inhibitors, increases gastric counts of bacteria normally present in the gastrointestinal tract. Treatment with PPIs may lead to slightly increased risk of gastrointestinal infections such as Salmonella and Campylobacter and possibly Clostridium difficile in hospitalized patients.
12.5 Pharmacogenomics
CYP2C19, a polymorphic enzyme, is involved in the metabolism of esomeprazole. The CYP2C19*1 allele is fully functional while the CYP2C19*2 and *3 alleles are nonfunctional. There are other alleles associated with no or reduced enzymatic function. Patients carrying two fully functional alleles are extensive metabolizers and those carrying two loss-of-function alleles are poor metabolizers. The systemic exposure to esomeprazole varies with a patient’s metabolism status: poor metabolizers > intermediate metabolizers > extensive metabolizers. Approximately 3% of Caucasians and 15 to 20% of Asians are CYP2C19 poor metabolizers.
Systemic esomeprazole exposures were modestly higher (approximately 17%) in CYP2C19 intermediate metabolizers (IM; n=6) compared to extensive metabolizers (EM; n=17) of CYP2C19. Similar pharmacokinetic differences were noted across these genotypes in a study of Chinese healthy subjects that included 7 EMs and 11 IMs. There is very limited pharmacokinetic information for poor metabolizers (PM) from these studies.
At steady state following once daily administration of esomeprazole 40 mg, the ratio of AUC in poor metabolizers to AUC in the rest of the population (EMs) is approximately 1.5. This change in exposure is not considered clinically meaningful.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of esomeprazole magnesium was assessed using studies of omeprazole, of which esomeprazole is an enantiomer. In two 24-month oral carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44, and 140.8 mg/kg/day (about 0.4 to 34 times the human dose of 40 mg/day expressed on a body surface area basis) produced gastric ECL cell carcinoids in a dose-related manner in both male and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood levels of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 3.4 times the human dose of 40 mg/day on a body surface area basis) for 1 year, then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of 1 year (94% treated vs. 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs. 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for 2 years. For this strain of rat no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret. A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive.
Esomeprazole was negative in the Ames mutation test, in the in vivo rat bone marrow cell chromosome aberration test, and the in vivo mouse micronucleus test. Esomeprazole, however, was positive in the in vitro human lymphocyte chromosome aberration test. Omeprazole was positive in the in vitro human lymphocyte chromosome aberration test, the in vivo mouse bone marrow cell chromosome aberration test, and the in vivo mouse micronucleus test.
The potential effects of esomeprazole on fertility and reproductive performance were assessed using omeprazole studies. Omeprazole at oral doses up to 138 mg/kg/day in rats (about 34 times the human dose of 40 mg/day on a body surface area basis) was found to have no effect on reproductive performance of parental animals.
13.2 Animal Toxicology and/or Pharmacology
Reproduction Studies
Reproduction studies have been performed in rats at oral doses up to 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 42 times an oral human dose of 40 mg on a body surface area basis) and have revealed no evidence of impaired fertility or harm to the fetus due to esomeprazole [see Use in Specific Populations ( 8.1) ].
Juvenile Animal Study
A 28-day toxicity study with a 14-day recovery phase was conducted in juvenile rats with esomeprazole magnesium at doses of 70 to 280 mg/kg/day (about 17 to 68 times a daily oral human dose of 40 mg on a body surface area basis). An increase in the number of deaths at the high dose of 280 mg/kg/day was observed when juvenile rats were administered esomeprazole magnesium from postnatal day 7 through postnatal day 35. In addition, doses equal to or greater than 140 mg/kg/day (about 34 times a daily oral human dose of 40 mg on a body surface area basis), produced treatment-related decreases in body weight (approximately 14%) and body weight gain, decreases in femur weight and femur length, and affected overall growth. Comparable findings described above have also been observed in this study with another esomeprazole salt, esomeprazole strontium, at equimolar doses of esomeprazole.
-
14 CLINICAL STUDIES
14.1 Healing of EE in Adults
The healing rates of esomeprazole magnesium delayed-release capsules 40 mg, esomeprazole magnesium delayed-release capsules 20 mg, and omeprazole delayed-release capsules 20 mg (the approved dose for this indication) once daily were evaluated in adult patients with endoscopically diagnosed EE in four multicenter, double-blind, randomized studies. The healing rates at Weeks 4 and 8 were evaluated and are shown in Table 11:
Table 11: EE Healing Rate (Life-Table Analysis) in Adults with EE Treated with Esomeprazole Magnesium Delayed-Release Capsules or Omeprazole Delayed-Release Capsules Once Daily in Four Clinical Studies
Study
No. of Patients
Treatment Group
EE Healing Rates
Significance Level 1
Week 4
Week 8
1
588
Esomeprazole magnesium delayed-release capsules 20 mg
68.7%
90.6%
N.S.
588
Omeprazole 20 mg
69.5%
88.3%
2
654
Esomeprazole magnesium delayed-release capsules 40 mg
75.9%
94.1%
p < 0.001
656
Esomeprazole magnesium delayed-release capsules 20 mg
70.5%
89.9%
p < 0.05
650
Omeprazole 20 mg
64.7%
86.9%
3
576
Esomeprazole magnesium delayed-release capsules 40 mg
71.5%
92.2%
N.S.
572
Omeprazole 20 mg
68.6%
89.8%
4
1216
Esomeprazole magnesium delayed-release capsules 40 mg
81.7%
93.7%
p < 0.001
1209
Omeprazole 20 mg
68.7%
84.2%
1log-rank test vs. omeprazole 20 mg.
N.S. = not significant (p > 0.05)
In these same studies of patients with EE, sustained heartburn resolution and time to sustained heartburn resolution were evaluated and are shown in Table 12:
Table 12: Sustained Resolution 1 of Heartburn in Adults with EE Treated with Esomeprazole Magnesium Delayed-Release Capsules or Omeprazole Delayed-Release Capsules Once Daily in Four Clinical Studies
Cumulative Percent 2 with Sustained Resolution
Study
No. of Patients
Treatment Group
Day 14
Day 28
Significance Level 3
1
573
Esomeprazole magnesium 20 mg
64.3%
72.7%
N.S.
555
Omeprazole 20 mg
64.1%
70.9%
2
621
Esomeprazole magnesium 40 mg
64.8%
74.2%
p <0.001
620
Esomeprazole magnesium 20 mg
62.9%
70.1%
N.S.
626
Omeprazole 20 mg
56.5%
66.6%
3
568
Esomeprazole magnesium 40 mg
65.4%
73.9%
N.S.
551
Omeprazole 20 mg
65.5%
73.1%
4
1187
Esomeprazole magnesium 40 mg
67.6%
75.1%
p <0.001
1188
Omeprazole 20 mg
62.5%
70.8%
1.Defined as 7 consecutive days with no heartburn reported in daily patient diary.
2.Defined as the cumulative proportion of patients who have reached the start of sustained resolution.
3.log-rank test vs. omeprazole 20 mg.
N.S. = not significant (p > 0.05)
In these four studies, the range of median days to the start of sustained resolution (defined as 7 consecutive days with no heartburn) was 5 days for esomeprazole magnesium 40 mg, 7 to 8 days for esomeprazole magnesium 20 mg and 7 to 9 days for omeprazole 20 mg.
There are no comparisons of 40 mg of esomeprazole magnesium with 40 mg of omeprazole in clinical trials assessing either healing or symptomatic relief of EE.
14.2 Maintenance of Healing of EE in Adults
Two multicenter, randomized, double-blind placebo-controlled 4-arm studies were conducted in adult patients with endoscopically confirmed, healed EE to evaluate esomeprazole magnesium 40 mg (n=174), 20 mg (n=180), 10 mg (n=168) or placebo (n=171) once daily over six months of treatment.
No additional clinical benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium delayed-release capsules 40 mg once daily is not a recommended regimen for the maintenance of healing of EE in adults.
The percentages of patients that maintained healing of EE at the various time points are shown in the Figures 2 and 3:
Figure 2: Maintenance of Healing Rates of EE in Adults by Month (Study 177)
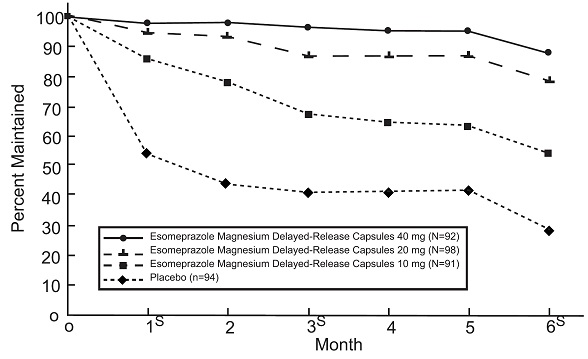
s= scheduled visit
Figure 3: Maintenance of EE Healing Rates in Adults by Month (Study 178)
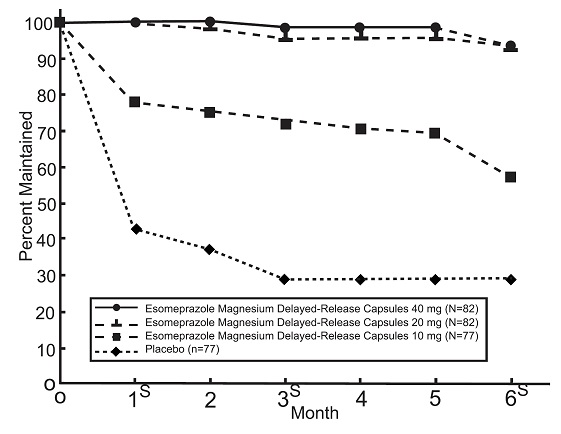
s= scheduled visit
Patients remained in remission significantly longer and the number of recurrences of EE was significantly less in patients treated with esomeprazole magnesium compared to placebo.
In both studies, the proportion of patients on esomeprazole magnesium who remained in remission and were free of heartburn and other GERD symptoms was well differentiated from placebo.
In a third multicenter open label study of 808 patients treated for 12 months with esomeprazole magnesium 40 mg, the percentage of patients that maintained healing of EE was 93.7% for six months and 89.4% for one year.
14.3 Symptomatic GERD in Adults
Two multicenter, randomized, double-blind, placebo-controlled studies were conducted in a total of 717 adult patients comparing four weeks of treatment with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily versus placebo for resolution of GERD symptoms. Patients had at least a 6-month history of heartburn episodes, no EE by endoscopy, and heartburn on at least four of the seven days immediately preceding randomization.
The percentage of patients that were symptom-free of heartburn was significantly higher in the esomeprazole magnesium groups compared to placebo at all follow-up visits (Weeks 1, 2, and 4).
No additional clinical benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium 40 mg once daily is not a recommended regimen for the treatment of symptomatic GERD in adults.
The percent of patients symptom-free of heartburn by day are shown in the Figures 4 and 5:
Figure 4: Percent of Patients Symptom-Free of Heartburn by Day (Study 225)
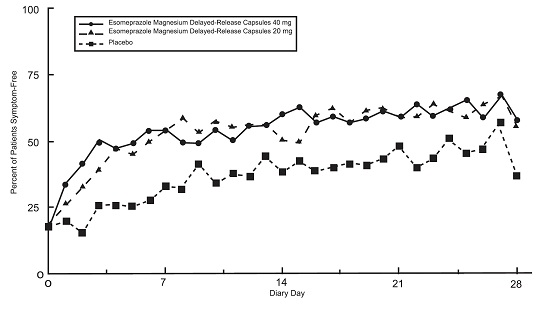
Figure 5: Percent of Patients Symptom-Free of Heartburn by Day (Study 226)
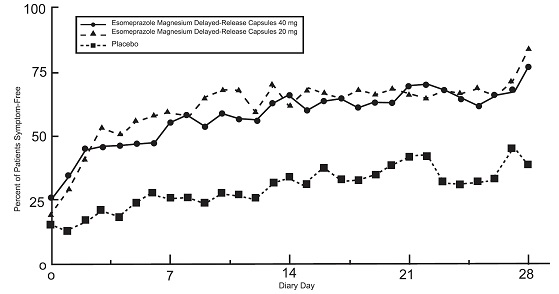
In three European symptomatic GERD trials, esomeprazole magnesium 20 mg and 40 mg and omeprazole 20 mg were evaluated. No significant treatment related differences were seen.
14.4 Pediatric GERD
12 Years to 17 Years of Age
In a multicenter, randomized, double-blind, parallel-group study, 149 adolescent patients (12 to 17 years of age; 89 female; 124 Caucasian, 15 Black, 10 Other) with clinically diagnosed GERD were treated with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily for up to 8 weeks to evaluate safety and tolerability. Patients were not endoscopically characterized as to the presence or absence of EE.
14.5 Risk Reduction of NSAID-Associated Gastric Ulcer
Two multicenter, double-blind, placebo-controlled studies were conducted in adult patients at risk of developing gastric and/or duodenal ulcers associated with continuous use of non-selective and COX-2 selective NSAIDs. A total of 1429 patients were randomized across the 2 studies. Patients ranged in age from 19 to 89 (median age 66 years) with 71% female, 29% male; 83% Caucasian, 5% Black, 4% Asian, and 8% Others. At baseline, the patients in these studies were endoscopically confirmed not to have ulcers but were determined to be at risk for ulcer occurrence due to their age (at least 60 years) and/or history of a documented gastric or duodenal ulcer within the past 5 years. Patients receiving NSAIDs and treated with esomeprazole magnesium delayed-release capsules 20 mg or 40 mg once daily experienced significant reduction in gastric ulcer occurrences relative to placebo treatment at 26 weeks. See Table 13. No additional benefit was seen with esomeprazole magnesium 40 mg over esomeprazole magnesium 20 mg. Esomeprazole magnesium 40 mg once daily is not a recommended regimen for the risk reduction of NSAID-associated gastric ulcer in adults. These studies did not demonstrate significant reduction in the development of NSAID-associated duodenal ulcer due to the low incidence.
Table 13: Cumulative Percentage of Patients at Least 60 Years of Age Taking NSAIDS Without Gastric Ulcers at 26 Weeks in Two Randomized Placebo-Controlled Studies
Study
No. of Patients
Treatment Group
% of Patients Remaining Gastric Ulcer Free 1
1
191
194
184
Esomeprazole magnesium delayed-release capsules 20 mg
Esomeprazole magnesium delayed-release capsules 40 mg
Placebo
95.4
96.7
88.2
2
267
271
257
Esomeprazole magnesium delayed-release capsules 20 mg
Esomeprazole magnesium delayed-release capsules 40 mg
Placebo
94.7
95.3
83.3
1%= Life Table Estimate. Significant difference from placebo (p<0.01).
14.6 H. pyloriEradication in Adult Patients with Duodenal Ulcer Disease
Two multicenter, randomized, double-blind studies were conducted in adult patients using a 10-day treatment regimen of triple therapy (esomeprazole magnesium, amoxicillin and clarithromycin). The first study (191) compared esomeprazole magnesium delayed-release capsules 40 mg once daily in combination with amoxicillin 1000 mg twice daily and clarithromycin 500 mg twice daily to esomeprazole magnesium delayed-release capsules 40 mg once daily plus clarithromycin 500 mg twice daily. The second study (193) compared esomeprazole magnesium delayed-release capsules 40 mg once daily in combination with amoxicillin 1000 mg twice daily and clarithromycin 500 mg twice daily to esomeprazole magnesium 40 mg once daily. H. pylori eradication rates, defined as at least two negative tests and no positive tests from CLOtest®, histology and/or culture, at 4 weeks post-therapy were significantly higher in the esomeprazole magnesium, amoxicillin and clarithromycin group than in the esomeprazole magnesium and clarithromycin group or the esomeprazole magnesium delayed-release capsules alone group. The results are shown in Table 14:
Table 14: H. pyloriEradication Rates at 4 Weeks after 10 Day Treatment Regimen % of Adult Patients Cured [95% Confidence Interval] (Number of Patients)
Study
Treatment Group
Per-Protocol 1
Intent-to-Treat 2
191
Esomeprazole magnesium, amoxicillin and clarithromycin
84% 3
[78, 89]
(n=196)
77% 3
[71, 82]
(n=233)
Esomeprazole magnesium and clarithromycin
55%
[48, 62]
(n=187)
52%
[45, 59]
(n=215)
193
Esomeprazole magnesium, amoxicillin and clarithromycin
85% 4
[74, 93]
(n=67)
78% 4
[67, 87]
(n=74)
Esomeprazole magnesium
5%
[0, 23]
(n=22)
4%
[0, 21]
(n=24)
1. Patients were included in the analysis if they had H. pylori infection documented at baseline, had at least one endoscopically verified duodenal ulcer ≥ 0.5 cm in diameter at baseline or had a documented history of duodenal ulcer disease within the past 5 years, and were not protocol violators. Patients who dropped out of the study due to an adverse reaction related to the study drug were included in the analysis as not H. pylori eradicated.
2. Patients were included in the analysis if they had documented H. pylori infection at baseline, had at least one documented duodenal ulcer at baseline, or had a documented history of duodenal ulcer disease, and took at least one dose of study medication. All dropouts were included as not H. pylori eradicated.
3. p < 0.05 compared to esomeprazole magnesium plus clarithromycin.
4.p < 0.05 compared to esomeprazole magnesium alone.
The percentage of patients with a healed baseline duodenal ulcer by 4 weeks after the 10-day treatment regimen in the esomeprazole magnesium delayed-release capsules, amoxicillin and clarithromycin group was 75% (n=156) and 57% (n=60) respectively, in the 191 and 193 studies (per-protocol analysis).
14.7 Pathological Hypersecretory Conditions, Including Zollinger-Ellison Syndrome, in Adults
In a multicenter, open-label dose-escalation study of 21 adult patients (15 males and 6 females, 18 Caucasian and 3 Black, mean age of 56 years) with pathological hypersecretory conditions, such as Zollinger-Ellison Syndrome, esomeprazole magnesium significantly inhibited gastric acid secretion. The initial dosage of esomeprazole magnesium delayed-release capsules was 40 mg twice daily in 19 patients and 80 mg twice daily in 2 patients. Total daily doses ranging from 80 mg to 240 mg for 12 months maintained gastric acid output below the target levels of 10 mEq/h in patients without prior gastric acid-reducing surgery and below 5 mEq/hr in patients with prior gastric acid-reducing surgery. At the Month 12 final visit, 18/20 (90%) patients had Basal Acid Output (BAO) under satisfactory control (median BAO = 0.17 mmol/hr). Of the 18 patients evaluated with a starting dose of esomeprazole magnesium 40 mg twice daily, 13 (72%) had their BAO controlled with the original dosing regimen at the final visit. See Table 15.
Table 15: Adequate Acid Suppression at Final Visit by Dosage Regimen in Adult Patients with Pathological Hypersecretory Conditions
Esomeprazole Magnesium dose at the Month 12 visit
BAO under adequate control at the Month 12 visit (N=20) 1
40 mg twice daily
13/15
80 mg twice daily
4/4
80 mg three times daily
1/1
1.One patient was not evaluated.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Esomeprazole magnesium delayed-release capsules USP, 20 mg are White opaque size ‘4’ hard gelatin capsule imprinted with “H” on cap and ‘E2’ on body filled with off white to pale yellow pellets.
Bottles of 30 Capsules NDC: 80425-0420-01
Bottles of 60 Capsules NDC: 80425-0420-02
Bottles of 90 Capsules NDC: 80425-0420-03
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep esomeprazole magnesium delayed-release capsules, USP container tightly closed.
Dispense in a tight container if the esomeprazole magnesium delayed-release capsules, USP product package is subdivided. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Acute Tubulointerstitial Nephritis
Advise the patient or caregiver to call the patient’s healthcare provider immediately if they experience signs and/or symptoms associated with suspected acute TIN [seeWarnings and Precautions ( 5.2) ].
Clostridium difficile-Associated Diarrhea
Advise the patient or caregiver to immediately call the patient’s healthcare provider if they experience diarrhea that does not improve [seeWarnings and Precautions ( 5.3) ].
Bone Fracture
Advise the patient or caregiver to report any fractures, especially of the hip, wrist or spine, to the patient’s healthcare provider [seeWarnings and Precautions ( 5.4) ].
Severe Cutaneous Adverse Reactions
Advise the patient or caregiver to discontinue esomeprazole magnesium delayed-release capsules and immediately call the patient’s healthcare provider for at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity signs or symptoms associated with Severe Cutaneous Adverse Reactions [seeWarnings and Precautions ( 5.5) ].
Cutaneous and Systemic Lupus Erythematosus
Advise the patient or caregiver to immediately call the patient’s healthcare provider for any new or worsening of symptoms associated with cutaneous or systemic lupus erythematosus [seeWarnings and Precautions ( 5.6) ].
Cyanocobalamin (Vitamin B-12) Deficiency
Advise the patient or caregiver to report any clinical symptoms that may be associated with cyanocobalamin deficiency to the patient’s healthcare provider if they have been receiving esomeprazole magnesium delayed-release capsules for longer than 3 years [seeWarnings and Precautions ( 5.8) ].
Hypomagnesemia and Mineral Metabolism
Advise the patient or caregiver to report any clinical symptoms that may be associated with hypomagnesemia, hypocalcemia, and/or hypokalemia to the patient’s healthcare provider, if they have been receiving esomeprazole magnesium delayed-release capsules for at least 3 months [seeWarnings and Precautions ( 5.9) ].
Drug Interactions
Advise the patient or caregiver to report to their healthcare provider if starting treatment with rilpivirine-containing products, clopidogrel, St. John’s Wort or rifampin; or, if they take high-dose methotrexate [seeContraindications ( 4) ,Warnings and Precautions ( 5.7,5.10,5.12)].
Administration
- Take esomeprazole magnesium delayed-release capsules at least one hour before meals.
- Antacids may be used concomitantly with esomeprazole magnesium delayed-release capsules.
- Swallow esomeprazole magnesium delayed-release capsules whole; do not chew or crush the capsules.
- For patients who have difficulty swallowing capsules, esomeprazole magnesium delayed-release capsules can be opened, and the contents sprinkled on applesauce. Use with other foods is not recommended.
1. Add one tablespoon of applesauce to an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
2. Open the esomeprazole magnesium delayed-release capsule and carefully empty the granules inside the capsule onto the applesauce.
3. Mix the granules with the applesauce.
4. Administer the mixture immediately. Do not chew or crush the granules.
5. Discard any remaining mixture. Do not store the mixture for future use.
- Esomeprazole magnesium delayed-release capsules can also be administered via a nasogastric tube, as described in the Instructions for Use.
Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By: HETERO TM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055, Indiaor
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313,
Telangana, India.Revised: 04/2024
Distributed by:
Advanced Rx of Tennessee, LLC
-
MEDICATION GUIDE
MEDICATION GUIDE
Esomeprazole Magnesium(es'' oh mep' ra zole mag nee' zee um)Delayed-Release Capsules, USPWhat is the most important information I should know about esomeprazole magnesium delayed-release capsules?
Esomeprazole magnesium delayed-release capsules may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your doctor.
Esomeprazole magnesium delayed-release capsules can cause serious side effects, including:- A type of kidney problem (acute tubulointerstitial nephritis). Some people who take proton pump inhibitor (PPI) medicines, including esomeprazole magnesium delayed-release capsules, may develop a kidney problem called acute tubulointerstitial nephritis that can happen at any time during treatment with esomeprazole magnesium delayed-release capsules. Call your doctor right away if you have a decrease in the amount that you urinate or if you have blood in your urine.
- Diarrhea caused by an infection (Clostridium difficile) in your intestines.Call your doctor right away if you have watery stools or stomach pain that does not go away. You may or may not have a fever.
- Bone fractures (hip, wrist, or spine).Bone fractures in the hip, wrist, or spine may happen in people who take multiple daily doses of PPI medicines and for a long period of time (a year or longer). Tell your doctor if you have a bone fracture, especially in the hip, wrist, or spine.
- Certain types of lupus erythematosus.Lupus erythematosus is an autoimmune disorder (the body’s immune cells attack other cells or organs in the body). Some people who take PPI medicines, including esomeprazole magnesium delayed-release capsules, may develop certain types of lupus erythematosus or have worsening of the lupus they already have. Call your doctor right away if you have new or worsening joint pain or a rash on your cheeks or arms that gets worse in the sun.
Esomeprazole magnesium delayed-release capsules can have other serious side effects. See “ What are the possible side effects of esomeprazole magnesium delayed-release capsules?”What are esomeprazole magnesium delayed-release capsules?
A prescription medicine called a proton pump inhibitor (PPI) used to reduce the amount of acid in your stomach.
Esomeprazole magnesium delayed-release capsules are used in adults for:- 4 to 8 weeks for the healing and symptom relief of acid-related damage to the esophagus (erosive esophagitis or EE). Your doctor may prescribe another 4-8 weeks of esomeprazole magnesium delayed-release capsules in patients whose EE does not heal.
- maintaining healing of EE.
- 4-8 weeks to treat heartburn and other symptoms that happen with gastroesophageal reflux disease (GERD).
- up to 6 months to reduce the risk of stomach ulcers in some people taking pain medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
- treating patients with a stomach infection ( Helicobacter pylori) and a stomach ulcer, along with the antibiotics amoxicillin and clarithromycin.
- the long-term treatment of conditions where your stomach makes too much acid, including Zollinger-Ellison Syndrome. Zollinger-Ellison Syndrome is a rare condition in which the stomach produces a more than normal amount of acid.
Esomeprazole magnesium delayed-release capsules are used in children and adolescents 12 to 17 years of age for: - 4 to 8 weeks to heal EE.
- 4 weeks to treat heartburn and other symptoms that happen with GERD.
Do not take esomeprazole magnesium delayed-release capsules if you are: - allergic to esomeprazole magnesium, any other PPI medicine, or any of the ingredients in esomeprazole magnesium delayed-release capsules. See the end of this Medication Guide for a complete list of ingredients in esomeprazole magnesium delayed-release capsules.
- rash
- face swelling
- throat tightness
- difficulty breathing
- taking a medicine that contains rilpivirine (EDURANT, COMPLERA, ODEFSEY) used to treat HIV-1 (Human Immunodeficiency Virus).
Before taking esomeprazole magnesium delayed-release capsules, tell your doctor about all of your medical conditions, including if you: - have low magnesium levels, low calcium levels and low potassium levels in your blood.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if esomeprazole magnesium delayed-release capsules will harm your unborn baby.
- are breastfeeding or planning to breastfeed. Esomeprazole magnesium may pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take esomeprazole magnesium delayed-release capsules.
Especially tell your doctor if you take:clopidogrel (Plavix), methotrexate (Otrxup, Rasuvo, Trexall, XATMEP), digoxin (LANOXIN), rilpivirine (EDURANT), St. John’s Wort (Hypericum perforatum), or rifampin (Rimactane, Rifater, Rifamate).How should I take esomeprazole magnesium delayed-release capsules? - Take esomeprazole magnesium delayed-release capsules exactly as prescribed by your doctor.
- Do not change your dose or stop esomeprazole magnesium delayed-release capsules without talking to your doctor.
- Take esomeprazole magnesium delayed-release capsules at least 1 hour before a meal.
- Antacids may be taken with esomeprazole magnesium delayed-release capsules.
- Swallow esomeprazole magnesium delayed-release capsules whole. Never chew or crush esomeprazole magnesium delayed-release capsules.
- If you have difficulty swallowing esomeprazole magnesium delayed-release capsules, you may open the capsule and empty the granules into 1 tablespoon of applesauce. The applesauce used should not be hot and should be soft enough to swallow without chewing. Do not mix the esomeprazole magnesium delayed-release capsules granules with any other food.
- Do not crush or chew the granules. Be sure to swallow the applesauce right away. Throw away any remaining mixture. Do not store it for later use.
- If you forget to take a dose of esomeprazole magnesium delayed-release capsules, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose on time. Do not take a double dose to make up for a missed dose.
- If you take too much esomeprazole magnesium delayed-release capsules, call your doctor or local poison control center right away at 1-800-222-1222, or go to the nearest hospital emergency room.
- See the Instructions for Useat the end of this Medication Guide for instructions, how to mix and give esomeprazole magnesium delayed-release capsules through a nasogastric tube.
What are the possible side effects of esomeprazole magnesium delayed-release capsules?
Esomeprazole magnesium delayed-release capsules can cause serious side effects, including:- See “What is the most important information I should know about esomeprazole magnesium delayed-release capsules?”
- Low vitamin B-12 levels in your bodycan happen in people who have taken esomeprazole magnesium delayed-release capsules for a long time (more than 3 years). Tell your doctor if you have symptoms of low vitamin B-12 levels, including shortness of breath, lightheadedness, irregular heartbeat, muscle weakness, pale skin, feeling tired, mood changes, and tingling or numbness in the arms and legs.
-
Low magnesium levels in your bodycan happen in people who have taken esomeprazole magnesium delayed-release capsules for at least 3 months.
Tell your doctor right away if you have symptoms of low magnesium levels, including seizures, dizziness, irregular heartbeat, jitteriness, muscle aches or weakness, and spasms of hands, feet or voice. - Stomach growths (fundic gland polyps).People who take PPI medicines for a long time have an increased risk of developing a certain type of stomach growths called fundic gland polyps, especially after taking PPI medicines for more than 1 year.
- Severe skin reactions.Esomeprazole magnesium delayed-release capsules can cause rare but severe skin reactions that may affect any part of your body. These serious skin reactions may need to be treated in a hospital and may be life threatening:
- Skin rash which may have blistering, peeling or bleeding on any part of your skin (including your lips, eyes, mouth, nose, genitals, hands or feet).
- You may also have fever, chills, body aches, shortness of breath, or enlarged lymph nodes.
The most common side effects of esomeprazole magnesium delayed-release capsules may include:- headache
- diarrhea
- nausea
- gas
- stomach (abdominal) pain
- constipation
- dry mouth
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store esomeprazole magnesium delayed-release capsules? - Store esomeprazole magnesium delayed-release capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the container of esomeprazole magnesium delayed-release capsules closed tightly.
General information about the safe and effective use of esomeprazole magnesium delayed-release capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use esomeprazole magnesium delayed-release capsules for a condition for which it was not prescribed. Do not give esomeprazole magnesium delayed-release capsules to other people, even if they have the same symptoms you have. They may harm them.
You can ask your pharmacist or doctor for information about esomeprazole magnesium delayed-release capsules that is written for health professionals.What are the ingredients in esomeprazole magnesium delayed-release capsules?
Active ingredient:esomeprazole magnesium trihydrate
Inactive ingredients in Esomeprazole Magnesium Delayed-Release Capsules (including the capsule shells):glyceryl monostearate, hydroxy propyl cellulose, hypromellose, magnesium stearate, methacrylic acid ethyl acrylate copolymer, polysorbate 80, simethicone, sugar spheres (contains sucrose and starch), talc and triethyl citrate. The capsule shells have the following inactive ingredients: gelatin, titanium dioxide and sodium lauryl sulfate.
The printing ink contains shellac, propylene glycol, strong ammonia solution, black iron oxide and potassium hydroxide.
Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
Manufactured by:
HETERO TM
HETERO LABS LIMITED
22-110, I.D.A., Jeedimetla,
Hyderabad – 500 055, India.
or
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313,
Telangana, India.
The brands listed are trademarks of their respective owners and are not trademarks of Hetero or Annora. The makers of these brands are not affiliated with and do not endorse Hetero or Annora or its products.
For more information, call 1-866-495-1995.This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 04/2024
Instructions for Use
Giving esomeprazole magnesium delayed-release capsules with water through a nasogastric tube (NG tube).
- Open the capsule and empty the granules into a 60 mL catheter tipped syringe. Mix with 50 mL of water. Use only a catheter tipped syringe to give esomeprazole magnesium delayed-release capsules through a NG tube.
- Replace the plunger and shake the syringe well for 15 seconds. Hold the syringe with the tip up and check for granules in the tip.
- Do not give the granules if they have dissolved or have broken into pieces.
- Attach the syringe to the NG tube. Give the medicine right away in the syringe through the NG tube into the stomach.
- After giving the granules, flush the NG tube with more water.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Medication Guide available at http://camberpharma.com/medication-guides
Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By: HETEROTM
Hetero Labs Limited
Jeedimetla, Hyderabad - 500 055,
Indiaor
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313,
Telangana, India.Distributed by:
Advanced Rx of Tennessee, LLC
The brands listed are trademarks of their respective owners and are not trademarks of Hetero or Annora. The makers of these brands are not affiliated with and do not endorse Hetero or Annora or its products.
Revised: 04/2024
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL 20mg Capsules 80425-0420
-
INGREDIENTS AND APPEARANCE
ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 80425-0420(NDC:31722-664) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM (UNII: R6DXU4WAY9) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) TALC (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SHELLAC (UNII: MB5IUD6JUA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) DIMETHICONE (UNII: 92RU3N3Y1O) Product Characteristics Color white Score no score Shape CAPSULE Size 14mm Flavor Imprint Code H;E2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80425-0420-3 90 in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2024 2 NDC: 80425-0420-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2024 3 NDC: 80425-0420-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211977 07/18/2024 Labeler - Advanced Rx of Tennessee, LLC (117023142) Establishment Name Address ID/FEI Business Operations Advanced Rx of Tennessee, LLC 117023142 repack(80425-0420)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


