OMNISCAN- gadodiamide injection
OMNISCAN by
Drug Labeling and Warnings
OMNISCAN by is a Prescription medication manufactured, distributed, or labeled by GE Healthcare Inc., GE Healthcare AS, GE Healthcare Ireland Limited, GE Healthcare Shanghai Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMNISCAN safely and effectively. See full prescribing information for OMNISCAN.
OMNISCAN™ (gadodiamide) Injection for Intravenous Use
Initial U.S. Approval: 1993WARNING: NOT FOR INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
See full prescribing information for complete boxed warning.
NOT FOR INTRATHECAL USE:
- Inadvertent intrathecal use of OMNISCAN has caused convulsions, coma, sensory and motor neurologic deficits (5.1).
NSF:
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities.
-
Do not administer OMNISCAN to patients with:
- chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- acute kidney injury (4).
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g., age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing (5.2).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
OMNISCAN is a gadolinium-based contrast agent for diagnostic magnetic resonance imaging (MRI) indicated for intravenous use to:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Sterile aqueous solution for intravenous injection; 287 mg/mL (3)
CONTRAINDICATIONS
Patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73m2) or acute kidney injury (4).
WARNINGS AND PRECAUTIONS
- Nephrogenic Systemic Fibrosis (NSF) has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeat dosing appears to increase the risk (5.2).
- Anaphylactoid and other serious hypersensitivity reactions including fatal reactions have occurred particularly in patients with history of allergy or drug reactions. Monitor patients closely for need of emergency cardiorespiratory support (5.3).
- Gadolinium is retained for months or years in brain, bone, and other organs. (5.4).
- Acute renal failure has occurred in patients with preexisting renal insufficiency. Use the lowest necessary dose of OMNISCAN and evaluate renal function in these patients (5.5).
ADVERSE REACTIONS
- The most frequent adverse reactions (≤ 3%) observed during OMNISCAN adult clinical studies were nausea, headache, and dizziness (6.1)
- Serious or life-threatening reactions include: cardiac failure, arrhythmia and myocardial infarction (6.1, 6.3)
To report SUSPECTED ADVERSE REACTIONS, contact GE Healthcare at 1-800-654-0118 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed (8.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
1 INDICATIONS AND USAGE
1.1 CNS (Central Nervous System)
1.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
2 DOSAGE AND ADMINISTRATION
2.1 CNS (Central Nervous System)
2.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
2.3 Dosage Chart
2.4 Dosing Guidelines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Not for Intrathecal Use
5.2 Nephrogenic Systemic Fibrosis
5.3 Hypersensitivity Reactions
5.4 Gadolinium Retention
5.5 Acute Renal Failure
5.6 Impaired Visualization of Lesions Detectable with Non-contrast MRI
5.7 Laboratory Test Findings
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience (Adults)
6.2 Clinical Studies Experience (Pediatrics)
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal/Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 CNS (Central Nervous System)
14.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
NOT FOR INTRATHECAL USE:
Inadvertent intrathecal use of OMNISCAN has caused convulsions, coma, sensory and motor neurologic deficits [see Warnings and Precautions (5.1)].
NSF:
- Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
-
Do not administer OMNISCAN to patients with:
- chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- acute kidney injury [see Contraindications (4)].
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g., age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- Do not exceed the recommended OMNISCAN dose and allow a sufficient period of time for elimination of the drug from the body prior to any readministration [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
1.1 CNS (Central Nervous System)
OMNISCAN is a gadolinium-based contrast agent indicated for intravenous use in MRI to visualize lesions with abnormal vascularity (or those thought to cause abnormalities in the blood-brain barrier) in the brain (intracranial lesions), spine, and associated tissues [see Clinical Studies (14.1)].
1.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
OMNISCAN is a gadolinium-based contrast agent indicated for intravenous use in MRI to facilitate the visualization of lesions with abnormal vascularity within the thoracic (noncardiac), abdominal, pelvic cavities, and the retroperitoneal space [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 CNS (Central Nervous System)
Adults: The recommended dose of OMNISCAN is 0.2 mL/kg (0.1 mmol/kg) administered as a bolus intravenous injection.
Pediatric Patients (2-16 years): The recommended dose of OMNISCAN is 0.2 mL/kg (0.1 mmol/kg) administered as a bolus intravenous injection [see Dosage and Administration (2.3)].
2.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
Adult and Pediatric Patients (2-16 years of age): For imaging the kidney, the recommended dose of OMNISCAN is 0.1 mL/kg (0.05 mmol/kg). For imaging the intrathoracic (noncardiac), intra-abdominal, and pelvic cavities, the recommended dose of OMNISCAN is 0.2 mL/kg (0.1 mmol/kg) [see Dosage and Administration (2.3)].
2.3 Dosage Chart
BODY WEIGHT PEDIATRIC ADULTS 0.05 0.1 0.05 0.1 kg lb (mmol/kg) (mmol/kg) VOLUME (mL) VOLUME (mL) - * The heaviest patient in clinical studies weighed 136 kg.
12 26 1.2 2.4 - - 14 31 1.4 2.8 - - 16 35 1.6 3.2 - - 18 40 1.8 3.6 - - 20 44 2 4 - - 22 48 2.2 4.4 - - 24 53 2.4 4.8 - - 26 57 2.6 5.2 - - 28 62 2.8 5.6 - - 30 66 3 6 - - 40 88 4 8 4 8 50 110 5 10 5 10 60 132 6 12 6 12 70 154 7 14 7 14 80 176 8 16 8 16 90 198 - - 9 18 100 220 - - 10 20 110 242 - - 11 22 120 264 - - 12 24 130* 286 - - 13 26 2.4 Dosing Guidelines
Inspect OMNISCAN visually for particulate matter and discoloration before administration, whenever solution and container permit. Do not use the solution if it is discolored or particulate matter is present.
Draw OMNISCAN into the syringe and use immediately. Discard any unused portion of OMNISCAN Injection.
To ensure complete delivery of the desired volume of contrast medium, follow the injection of OMNISCAN with a 5 mL flush of 0.9% sodium chloride, as provided in the Prefill Plus needle-free system. Complete the imaging procedure within 1 hour of administration of OMNISCAN.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Not for Intrathecal Use
Inadvertent intrathecal use of OMNISCAN has occurred and caused convulsions, coma, sensory and motor neurologic deficits.
5.2 Nephrogenic Systemic Fibrosis
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR < 30 mL/min/1.73m2) as well as patients with acute kidney injury. Do not administer OMNISCAN to these patients. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following OMNISCAN administration to GE Healthcare (1-800-654-0118) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. When administering a GBCA, do not exceed the recommended dose and allow a sufficient period of time for elimination of the agent prior to any readministration [see Boxed Warning, Contraindications (4), Clinical Pharmacology (12.2) and Dosage and Administration (2)].
5.3 Hypersensitivity Reactions
Anaphylactoid and anaphylactic reactions, with cardiovascular, respiratory and/or cutaneous manifestations, resulting in death have occurred. Personnel trained in resuscitation techniques and resuscitation equipment should be present prior to OMNISCAN administration. If a hypersensitivity reaction occurs, stop OMNISCAN Injection and immediately begin appropriate therapy. Observe patients closely, particularly those with a history of drug reactions, asthma, allergy or other hypersensitivity disorders, during and up to several hours after OMNISCAN Injection.
5.4 Gadolinium Retention
Gadolinium is retained for months or years in several organs. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (e.g. brain, skin, kidney, liver, and spleen). The duration of retention also varies by tissue and is longest in bone. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, gadolinium retention varies among the linear agents with Omniscan (gadodiamide) and Optimark (gadoversetamide) causing greater retention than other linear agents [Eovist (gadoxetate disodium), Magnevist (gadopentetate dimeglumine), MultiHance (gadobenate dimeglumine)]. Retention is lowest and similar among the macrocyclic GBCAs [Dotarem (gadoterate meglumine), Gadavist (gadobutrol), ProHance (gadoteridol)].
Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function [see Warnings and Precautions (5.2)]. There are rare reports of pathologic skin changes in patients with normal renal function. Adverse events involving multiple organ systems have been reported in patients with normal renal function without an established causal link to gadolinium retention [see Adverse Reactions (6.3)].
While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients [see Use in Specific Populations (8.1, 8.4)], and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies, particularly closely spaced studies when possible.
5.5 Acute Renal Failure
In patients with renal insufficiency, acute renal failure requiring dialysis or worsening renal function have occurred, mostly within 48 hours of OMNISCAN Injection. The risk of renal failure may increase with increasing dose of gadolinium contrast. Use the lowest necessary dose of contrast and evaluate renal function in patients with renal insufficiency. Acute renal failure was observed in < 1% of patients in OMNISCAN clinical studies [see Adverse Reactions (6)].
OMNISCAN is cleared by glomerular filtration. Hemodialysis also enhances OMNISCAN clearance [see Use in Specific Populations (8.5, 8.6)].
5.6 Impaired Visualization of Lesions Detectable with Non-contrast MRI
Paramagnetic contrast agents such as OMNISCAN might impair the visualization of lesions which are seen on the non-contrast MRI. This may be due to effects of the paramagnetic contrast agent, or imaging parameters. Exercise caution when OMNISCAN MRI scans are interpreted in the absence of a companion non-contrast MRI.
5.7 Laboratory Test Findings
Asymptomatic, transitory changes in serum iron have been observed. The clinical significance is unknown.
OMNISCAN interferes with serum calcium measurements with some colorimetric (complexometric) methods commonly used in hospitals, resulting in serum calcium concentrations lower than the true values. In patients with normal renal function, this effect lasts for 12-24 hours. In patients with decreased renal function, the interference with calcium measurements is expected to last during the prolonged elimination of OMNISCAN. After patients receive OMNISCAN, careful attention should be used in selecting the type of method used to measure calcium.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Nephrogenic systemic fibrosis [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.3)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Clinical Studies Experience (Adults)
In clinical studies 1160 patients were exposed to OMNISCAN. The most frequent adverse reactions were nausea, headache, and dizziness that occurred in 3% or less of the patients. The majority of these reactions were of mild to moderate intensity.
The following adverse reactions occurred in 1% or less of patients:
Application Site Disorders: Injection site reaction.
Autonomic Nervous System Disorders: Vasodilation.
Body as a Whole-General Disorders: Anaphylactoid reactions (characterized by cardiovascular, respiratory, and cutaneous symptoms), fever, hot flushes, rigors, fatigue, malaise, pain, syncope.
Cardiovascular Disorders: Cardiac failure, rare arrhythmia and myocardial infarction resulting in death in patients with ischemic heart disease, flushing, chest pain, deep thrombophlebitis.
Central and Peripheral Nervous System Disorders: Convulsions including grand mal, ataxia, abnormal coordination, paresthesia, tremor, aggravated multiple sclerosis (characterized by sensory and motor disturbances), aggravated migraine.
Gastrointestinal System Disorders: Abdominal pain, diarrhea, eructation, dry mouth/vomiting, melena.
Hearing and Vestibular Disorders: Tinnitus.
Liver and Biliary System Disorders: Abnormal hepatic function.
Musculoskeletal System Disorders: Arthralgia, myalgia.
Respiratory System Disorders: Rhinitis, dyspnea.
Skin and Appendage Disorders: Pruritus, rash, erythematous rash, sweating increased, urticaria.
Special Senses, Other Disorders: Taste loss, taste perversion.
Urinary System Disorders: Acute reversible renal failure.
Vision Disorders: Abnormal vision.
6.2 Clinical Studies Experience (Pediatrics)
In the 97 pediatric patients in CNS studies with OMNISCAN [see Clinical Studies (14.1)] and the 144 pediatric patients in published literature, the adverse reactions were similar to those reported in adults.
6.3 Postmarketing Experience
Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been identified during the postmarketing use of OMNISCAN:
Nervous System Disorders: Inadvertent intrathecal use causes convulsions, coma, paresthesia, paresis. Convulsions have also been reported with intravenous use in patients with and without a history of convulsions or brain lesions.
General Disorders: Nephrogenic Systemic Fibrosis (NSF) [see Warnings and Precautions (5.2)].
Adverse events with variable onset and duration have been reported after GBCA administration [see Warnings and Precautions (5.4)]. These include fatigue, asthenia, pain syndromes, and heterogeneous clusters of symptoms in the neurological, cutaneous, and musculoskeletal systems.
Renal and Urinary System Disorders: In patients with pre-existing renal insufficiency: acute renal failure, renal impairment, blood creatinine increased [see Warnings and Precautions (5.5)].
Skin: Gadolinium associated plaques.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
GBCAs cross the placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes are limited and inconclusive. Because of the potential risks of gadolinium to the fetus, use OMNISCAN only if imaging is essential during pregnancy and cannot be delayed.
Contrast enhancement is visualized in the human placenta and fetal tissues after maternal GBCA administration.
Cohort studies and case reports on exposure to GBCAs during pregnancy have not reported a clear association between GBCAs and adverse effects in the exposed neonates. However, a retrospective cohort study, comparing pregnant women who had a GBCA MRI to pregnant women who did not have an MRI, reported a higher occurrence of stillbirths and neonatal deaths in the group receiving GBCA MRI. Limitations of this study include a lack of comparison with non-contrast MRI and lack of information about the maternal indication for MRI. Overall, these data preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of GBCAs in pregnancy.
GBCAs administered to pregnant non-human primates (0.1 mmol/kg on gestational days 85 and 135) result in measurable gadolinium concentration in the offspring in bone, brain, skin, liver, kidney, and spleen for at least 7 months. GBCAs administered to pregnant mice (2 mmol/kg daily on gestational days 16 through 19) result in measurable gadolinium concentrations in the pups in bone, brain, kidney, liver, blood, muscle, and spleen at one month postnatal age.
OMNISCAN has been shown to have an adverse effect on embryo-fetal development in rabbits at dosages as low as 0.5 mmol/kg/day for 13 days during gestation (approximately 0.6 times the human dose based on a body surface area comparison). These adverse effects are observed as an increased incidence of flexed appendages and skeletal malformations which may be due to maternal toxicity since the body weight of the dams was reduced in response to OMNISCAN administration during pregnancy. In rat studies, fetal abnormalities were not observed at doses up to 2.5 mmol/kg/day for 10 days during gestation (1.3 times the maximum human dose based on a body surface area comparison); however, maternal toxicity was not achieved in these studies and a definitive conclusion about teratogenicity in rats at doses above 2.5 mmol/kg/day cannot be made.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, exercise caution when administering OMNISCAN to a nursing woman.
8.4 Pediatric Use
The safety and efficacy of OMNISCAN at a single dose of 0.05 to 0.1 mmol/kg have been established in pediatric patients over 2 years of age based on adequate and well controlled studies of OMNISCAN in adults, a pediatric CNS imaging study, and safety data in the scientific literature. However, the safety and efficacy of doses greater than 0.1 mmol/kg and of repeated doses have not been studied in pediatric patients.
Pharmacokinetics of OMNISCAN have not been studied in pediatrics. The glomerular filtration rate of neonates and infants is much lower than that of adults. The pharmacokinetics volume of distribution is also different. Therefore, the optimal dosing regimen and imaging times in patients under 2 years of age have not been established.
8.5 Geriatric Use
In clinical studies of OMNISCAN, 243 patients were between 65 and 80 years of age while 15 were over 80. No overall differences in safety or effectiveness were observed between these patients and younger patients. Other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity in the elderly cannot be ruled out. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
OMNISCAN is excreted by the kidney, and the risk of toxic reactions to OMNISCAN may be greater in patients with impaired renal function [see Warnings and Precautions (5.4)]. Because elderly patients are more likely to have decreased renal function, select dose carefully and consider assessment of renal function before OMNISCAN use.
8.6 Renal/Hepatic Impairment
Dose adjustments in renal or hepatic impairment have not been studied. Caution should be exercised in patients with impaired renal insufficiency [see Warnings and Precautions (5.2, 5.5)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
OMNISCAN (gadodiamide) Injection is the formulation of the gadolinium complex of diethylenetriamine pentaacetic acid bismethylamide, and is an injectable, nonionic extracellular enhancing agent for magnetic resonance imaging. OMNISCAN is administered by intravenous injection.
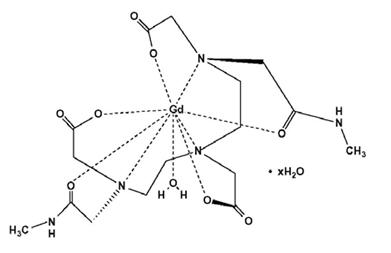
OMNISCAN is provided as a sterile, clear, colorless to slightly yellow, aqueous solution. Each 1 mL contains 287 mg gadodiamide and 12 mg caldiamide sodium in Water for Injection. The pH is adjusted between 5.5 and 7.0 with hydrochloric acid and/or sodium hydroxide. OMNISCAN contains no antimicrobial preservative. OMNISCAN is a 0.5 mol/L solution of aqua[5,8-bis(carboxymethyl)-11-[2-(methylamino)-2-oxoethyl]-3-oxo-2,5,8,11-tetraazatridecan-13-oato (3-)-N5, N8, N11, O3, O5, O8, O11, O13] gadolinium hydrate, with a molecular weight of 573.66 (anhydrous), an empirical formula of C16H28GdN5O9∙xH2O, and the following structural formula:
Pertinent physicochemical data for OMNISCAN are noted below:
PARAMETER Osmolality (mOsmol/kg water) @ 37°C 789 Viscosity (cP) @ 20°C 2 @ 37°C 1.4 Density (g/mL) @ 25°C 1.14 Specific gravity @ 25°C 1.15 OMNISCAN has an osmolality approximately 2.8 times that of plasma at 37°C and is hypertonic under conditions of use.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
In magnetic resonance imaging, visualization of normal and pathologic tissue depends in part on variations in the radiofrequency signal intensity. These variations occur due to: changes in proton density; alteration of the spin-lattice or longitudinal relaxation time (T1); and variation of the spin-spin or transverse relaxation time (T2). OMNISCAN is a paramagnetic agent with unpaired electron spins which generate a local magnetic field. As water protons move through this local magnetic field, the changes in magnetic field experienced by the protons reorient them with the main magnetic field more quickly than in the absence of a paramagnetic agent.
By increasing the relaxation rate, OMNISCAN decreases both the T1 and T2 relaxation times in tissues where it is distributed. At clinical doses, the effect is primarily on the T1 relaxation time, and produces an increase in signal intensity. Disruption of the blood-brain barrier or abnormal vascularity allows accumulation of OMNISCAN in lesions such as neoplasms, abscesses, and subacute infarcts. The pharmacokinetic parameters of OMNISCAN in various lesions are not known.
12.3 Pharmacokinetics
The pharmacokinetics of intravenously administered gadodiamide in normal subjects conforms to an open, two-compartment model with mean distribution and elimination half-lives (reported as mean ± SD) of 3.7 ± 2.7 minutes and 77.8 ± 16 minutes, respectively. Gadodiamide is eliminated primarily in the urine with 95.4 ± 5.5% (mean ± SD) of the administered dose eliminated by 24 hours. The renal and plasma clearance rates of gadodiamide are nearly identical (1.7 and 1.8 mL/min/kg, respectively), and are similar to that of substances excreted primarily by glomerular filtration. The volume of distribution of gadodiamide (200 ± 61 mL/kg) is equivalent to that of extracellular water. Gadodiamide does not bind to human serum proteins in vitro. Following GBCA administration, gadolinium is present for months or years in brain, bone, skin, and other organs [see Warnings and Precautions (5.4)]. Pharmacokinetic and pharmacodynamic studies have not been systematically conducted to determine the optimal dose and imaging time in patients with abnormal renal function or renal failure, in the elderly, or in pediatric patients with immature renal function.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term animal studies have not been performed to evaluate the carcinogenic potential of gadodiamide. The results of the following genotoxicity assays were negative: in vitro bacterial reverse mutation assay, in vitro Chinese Hamster Ovary (CHO)/Hypoxanthine Guanine Phosphoribosyl Transferase (HGPT) forward mutation assay, in vitro CHO chromosome aberration assay, and the in vivo mouse micronucleus assay at intravenous doses of 27 mmol/kg (approximately 7 times the maximum human dose based on a body surface area comparison). Impairment of male or female fertility was not observed in rats after intravenous administration three times per week at the maximum dose tested of 1.0 mmol/kg (approximately 0.5 times the maximum human dose based on a body surface area comparison).
-
14 CLINICAL STUDIES
14.1 CNS (Central Nervous System)
OMNISCAN (0.1 mmol/kg) contrast enhancement in CNS MRI was evident in a study of 439 adults. In a study of sequential dosing, 57 adults received OMNISCAN 0.1 mmol/kg followed by 0.2 mmol/kg within 20 minutes (for cumulative dose of 0.3 mmol/kg). The MRIs were compared blindly. In 54/56 (96%) patients, OMNISCAN contrast enhancement was evident with both the 0.1 mmol/kg and cumulative 0.3 mmol/kg OMNISCAN doses relative to non-contrast MRI.
In comparison to the non-contrast MRI, increased numbers of brain and spine lesions were noted in 42% of patients who received OMNISCAN at any dose. In comparisons of 0.1 mmol/kg versus 0.3 mmol/kg, the results were comparable in 25/56 (45%); in 1/56 (2%) OMNISCAN 0.1 mmol/kg dose provided more diagnostic value and in 30/56 (54%) the cumulative OMNISCAN 0.3 mmol/kg dose provided more diagnostic value.
The usefulness of a single 0.3 mmol/kg bolus in comparison to the cumulative 0.3 mmol/kg (0.1 mmol/kg followed by 0.2 mmol/kg) has not been established.
OMNISCAN as a single 0.1 mmol/kg dose was evaluated in 97 pediatric patients with a mean age of 8.9 (2-18) years referred for CNS MRI. Postcontrast MRI provided added diagnostic information, diagnostic confidence, and new patient management information in 76%, 67%, and 52%, respectively, of pediatrics.
14.2 Body (Intrathoracic [noncardiac], Intra-abdominal, Pelvic and Retroperitoneal Regions)
OMNISCAN was evaluated in a controlled trial of 276 patients referred for body MRI. These patients had a mean age of 57 (9-88) years. Patients received 0.1 mmol/kg OMNISCAN for imaging the thorax (noncardiac), abdomen, and pelvic organs, or a dose of 0.05 mmol/kg for imaging the kidney. Pre- and post-OMNISCAN images were evaluated blindly for the degree of diagnostic value rated on a scale of "remarkably improved, improved, no change, worse, and cannot be determined." The postcontrast results showed "remarkably improved" or "improved" diagnostic value in 90% of the thorax, liver, and pelvis patients, and in 95% of the kidney patients.
In a dose ranging study 258 patients referred for body MRI received OMNISCAN 0.025, 0.05, 0.1 mmol/kg. The lowest effective dose of OMNISCAN for the kidney was 0.05 mmol/kg.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OMNISCAN (gadodiamide) Injection is a sterile, clear, colorless to slightly yellow, aqueous solution containing 287 mg/mL of gadodiamide in rubber stoppered vials and polypropylene syringes. OMNSICAN is supplied in the following sizes:
5 mL fill in 10 mL vial, box of 10 (NDC: 0407-0690-05)
10 mL vial, box of 10 (NDC: 0407-0690-10)
15 mL fill in 20 mL vial, box of 10 (NDC: 0407-0690-15)
20 mL vial, box of 10 (NDC: 0407-0690-20)
10 mL fill in 20 mL prefilled syringe, box of 10 (NDC: 0407-0690-12)
15 mL fill in 20 mL prefilled syringe, box of 10 (NDC: 0407-0690-17)
20 mL prefilled syringe, box of 10 (NDC: 0407-0690-22)Prefill Plus™ needle-free system
OMNISCAN 15 mL, box of 10 (NDC: 0407-0691-62)
Contains: OMNISCAN 15 mL fill in 20 mL Single Dose Prefilled Syringe and 5 mL 0.9% Sodium Chloride Injection, USP I.V. Flush SyringePrefill Plus™ needle-free system
OMNISCAN 20 mL, box of 10 (NDC: 0407-0691-63)
Contains: OMNISCAN 20 mL fill in 20 mL Single Dose Prefilled Syringe and 5 mL 0.9% Sodium Chloride Injection, USP I.V. Flush SyringeProtect OMNISCAN from strong daylight and direct exposure to sunlight. Do not freeze. Freezing can cause small cracks in the vials, which would compromise the sterility of the product. Do not use if the product is inadvertently frozen.
Store OMNISCAN at controlled room temperature 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Patients receiving OMNISCAN should be instructed to inform their physician if they:
- are pregnant or breast feeding, or
- have a history of renal and/or liver disease, convulsions, asthma or allergic respiratory disorders, or recent administration of gadolinium-based contrast.
GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. To counsel patients at risk for NSF:
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following OMNISCAN administration such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain deep in the hip bones or ribs; or muscle weakness.
Gadolinium Retention
- Advise patients that gadolinium is retained for months or years in brain, bone, skin, and other organs in patients with normal renal function. The clinical consequences of retention are unknown. Retention depends on multiple factors and is greater following administration of linear GBCAs than following administration of macrocyclic GBCAs [see Warnings and Precautions (5.4)].
-
SPL UNCLASSIFIED SECTION
Distributed by GE Healthcare Inc. Marlborough, MA 01752 U.S.A.
Manufactured by GE Healthcare Ireland Limited, Cork, IrelandProduct of Norwegian Origin.
OMNISCAN is a trademark of General Electric Company or one of its subsidiaries.
GE and the GE Monogram are trademarks of General Electric Company.
© 2018 General Electric Company – All rights reserved.
ONC-2Z-CORK -
MEDICATION GUIDE
MEDICATION GUIDE
OMNISCAN™ (OMNI-scan)
(gadodiamide)
Injection for intravenous useWhat is OMNISCAN? - OMNISCAN is a prescription medicine called a gadolinium-based contrast agent (GBCA). OMNISCAN, like other GBCA medicines, is used with a magnetic resonance imaging (MRI) scanner.
- An MRI exam with a GBCA, including OMNISCAN, helps your doctor to see problems better than an MRI exam without a GBCA.
- Your doctor has reviewed your medical records and has determined that you would benefit from using a GBCA with your MRI exam.
What is the most important information I should know about OMNISCAN? - OMNISCAN contains a metal called gadolinium. Small amounts of gadolinium can stay in your body including the brain, bones, skin and other parts of your body for a long time (several months to years).
- It is not known how gadolinium may affect you, so far, studies have not found harmful effects in patients with normal kidneys.
- Rarely, patients have reported pains, tiredness, and skin, muscle or bone ailments for a long time, but these symptoms have not been directly linked to gadolinium.
- There are different GBCAs that can be used for your MRI exam. The amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after Omniscan or Optimark than after Eovist, Magnevist, or MultiHance. Gadolinium stays in the body the least after Dotarem, Gadavist, or ProHance.
- People who get many doses of gadolinium medicines, women who are pregnant and young children may be at increased risk from gadolinium staying in the body.
- Some people with kidney problems who get gadolinium medicines can develop a condition with severe thickening of the skin, muscles and other organs in the body (nephrogenic systemic fibrosis). Your healthcare provider should screen you to see how well your kidneys are working before you receive OMNISCAN.
Do not receive OMNISCAN if you have had a severe allergic reaction to OMNISCAN. Before receiving OMNISCAN, tell your healthcare provider about all your medical conditions, including if you: - have had any MRI procedures in the past where you received a GBCA. Your healthcare provider may ask you for more information including the dates of these MRI procedures.
- are pregnant or plan to become pregnant. It is not known if OMNISCAN can harm your unborn baby. Talk to your healthcare provider about the possible risks to an unborn baby if a GBCA such as OMNISCAN is received during pregnancy
- have kidney problems, diabetes, or high blood pressure.
- have had an allergic reaction to dyes (contrast agents) including GBCAs
What are the possible side effects of OMNISCAN? - See "What is the most important information I should know about OMNISCAN?"
- Allergic reactions. OMNISCAN can cause allergic reactions that can sometimes be serious. Your healthcare provider will monitor you closely for symptoms of an allergic reaction.
These are not all the possible side effects of OMNISCAN.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of OMNISCAN.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about OMNISCAN that is written for health professionals.What are the ingredients in OMNISCAN?
Active ingredient: gadodiamide
Inactive ingredients: caldiamide sodium, hydrochloric acid, sodium hydroxide
Manufactured by: GE Healthcare AS Oslo, Norway or GE Healthcare Ireland or GE Healthcare (Shanghai) Co., Ltd
OMNISCAN is a trademark of General Electric Company or one of its subsidiaries. GE and the GE Monogram are trademarks of General Electric Company.
For more information, go to www.GEHealthcare.com or call 1-800-654-0118.This Medication Guide has been approved by the U.S. Food and Drug Administration 04/2018 - PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
-
PRINCIPAL DISPLAY PANEL - 15 mL Syringe Box Label
GE Healthcare
OMNISCAN™
(gadodiamide) InjectionPrefill Plus™ needle-free system
15 mL
287 mg/mLNDC 0407-0691-62
CONTAINS:
1 — Omniscan™ 15 mL fill in 20 mL Single Dose Prefilled Syringe
1 — 5 mL 0.9% Sodium Chloride (0.9% NaCl) I.V. Flush Syringe
OMNISCAN (gadodiamide) Injection – Each 1 mL contains 287 mg gadodiamide, 12 mg
caldiamide sodium, in Water for Injection. The pH is adjusted between 5.5 and 7.0 with
hydrochloric acid or sodium hydroxide.
No preservative added. For intravenous use only. Each syringe for one procedure only.
Discard unused portion.
O.9% Sodium Chloride (0.9% NaCl) – Each Sterile Flush Syringe contains a sterile isotonic
solution of 0.9% Sodium Chloride (0.9% NaCl). Each mL contains 9 mg sodium chloride.
The osmolarity is 0.308 mOsmol/mL (calc.). Preservative free. For I.V. flush only.Do not use if solution is discolored or contains a precipitate.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP].
Protect from light. Do not freeze. For indications and dosage, see package insert. Rx ONLY.Exp.:
Lot:
1192196 USA

-
INGREDIENTS AND APPEARANCE
OMNISCAN
gadodiamide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-0690 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength gadodiamide (UNII: 84F6U3J2R6) (gadodiamide - UNII:84F6U3J2R6) gadodiamide 287 mg in 1 mL Inactive Ingredients Ingredient Name Strength caldiamide sodium (UNII: 1M6415C70M) 12 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (Clear, colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-0690-05 10 in 1 BOX 04/19/2002 1 5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0407-0690-10 10 in 1 BOX 04/19/2002 2 10 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 0407-0690-15 10 in 1 BOX 04/19/2002 3 15 mL in 1 VIAL; Type 0: Not a Combination Product 4 NDC: 0407-0690-20 10 in 1 BOX 04/19/2002 4 20 mL in 1 VIAL; Type 0: Not a Combination Product 5 NDC: 0407-0690-12 10 in 1 BOX 04/19/2002 5 10 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product 6 NDC: 0407-0690-17 10 in 1 BOX 04/19/2002 6 15 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product 7 NDC: 0407-0690-22 10 in 1 BOX 04/19/2002 7 20 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020123 04/19/2002 OMNISCAN
gadodiamide injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0407-0691 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength gadodiamide (UNII: 84F6U3J2R6) (gadodiamide - UNII:84F6U3J2R6) gadodiamide 287 mg in 1 mL Inactive Ingredients Ingredient Name Strength caldiamide sodium (UNII: 1M6415C70M) 12 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW (Clear, colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0407-0691-62 10 in 1 BOX 09/21/2006 1 15 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0407-0691-63 10 in 1 BOX 09/21/2006 2 20 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020123 09/21/2006 Labeler - GE Healthcare Inc. (053046579) Establishment Name Address ID/FEI Business Operations GE Healthcare AS 515048908 MANUFACTURE(0407-0690, 0407-0691) , REPACK(0407-0690, 0407-0691) , RELABEL(0407-0690, 0407-0691)
Trademark Results [OMNISCAN]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.