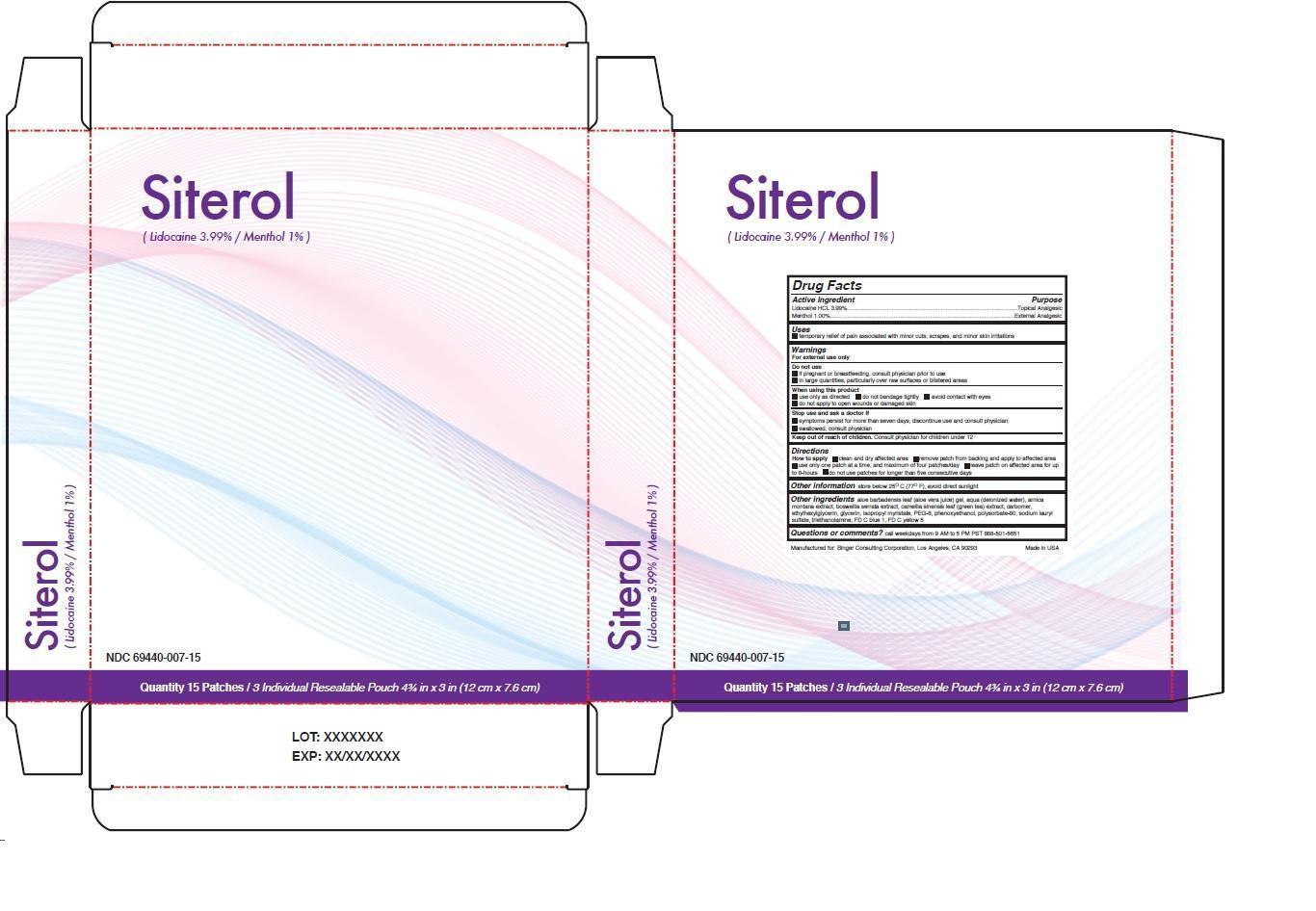
SITEROL- lidocaine hcl, menthol patch
Siterol by
Drug Labeling and Warnings
Siterol by is a Otc medication manufactured, distributed, or labeled by Binger Consulting Corporation, Pocono Coated Products, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Warnings
For external use only
Do not use
if pregnant or breastfeeding, consult physician prior to use
in large quantities, particularly over raw surfaces or blistered areas
When using this product
use only as directed do not bandage tightly avoid contact with eyes
do not apply to open wounds or damaged skin
Stop use and ask a doctor if
symptoms persist for more than seven days, discontinue use and consult physician
swallowed, consult physician
- Directions
-
INACTIVE INGREDIENT
Other ingredients aloe barbadensis leaf (aloe vera juice) gel, aqua (deionized water), arnica montana extract, boswellia serrata extract, camellia sinensis leaf (green tea) extract, carbomer, ethylhexylglycerin, glycerin, isopropyl myristate, PEG-8, phenoxyethanol, polysorbate-80, sodium lauryl sulfate, triethanolamine, FD C blue 1, FD C yellow 5
- QUESTIONS
- STORAGE AND HANDLING
- Packaging
-
INGREDIENTS AND APPEARANCE
SITEROL
lidocaine hcl, menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69440-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 3.99 g in 100 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ARNICA MONTANA (UNII: O80TY208ZW) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALOE VERA LEAF (UNII: ZY81Z83H0X) BOSWELLIA SACRA WHOLE (UNII: 8O600AZL0W) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) PEG-8 GLYCERYL ISOSTEARATE (UNII: 74QQ5X3KL1) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TRIETHANOLAMINE BENZOATE (UNII: M3EN4GC19W) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69440-007-15 15 in 1 BOX 01/01/2015 1 100 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 01/01/2015 Labeler - Binger Consulting Corporation (079635976) Establishment Name Address ID/FEI Business Operations Pocono Coated Products, LLC 362785771 manufacture(69440-007)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.