These highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE TABLETS and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METFORMIN HYDROCHLORIDE TABLETS and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS. METFORMIN HYDROCHLORIDE tablets, for oral use METFORMIN HYDROCHLORIDE extended-release tablets, for oral use Initial U.S. Approval: 1995
METFORMIN HYDROCHLORIDE by
Drug Labeling and Warnings
METFORMIN HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by PD-Rx Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
METFORMIN HYDROCHLORIDE- metformin hydrochloride tablet, film coated
PD-Rx Pharmaceuticals, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METFORMIN HYDROCHLORIDE TABLETS and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for METFORMIN HYDROCHLORIDE TABLETS and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS.
METFORMIN HYDROCHLORIDE tablets, for oral use METFORMIN HYDROCHLORIDE extended-release tablets, for oral use Initial U.S. Approval: 1995 WARNING: LACTIC ACIDOSISSee full prescribing information for complete boxed warning.
INDICATIONS AND USAGEMetformin hydrochloride tablets are biguanide indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus. ( 1) Metformin hydrochloride extended-release tablets are biguanide indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. ( 1) DOSAGE AND ADMINISTRATIONAdult Dosage for Metformin Hydrochloride Tablets:
Adult Dosage for Metformin Hydrochloride Extended-Release Tablets:
Pediatric Dosage for Metformin Hydrochloride Tablets:
Renal Impairment:
Discontinuation for Iodinated Contrast Imaging Procedures:
DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSFor Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, the most common adverse reactions (>5.0%) are diarrhea, nausea/vomiting, flatulence, asthenia, indigestion, abdominal discomfort, and headache. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 7/2018 |
FULL PRESCRIBING INFORMATION
WARNING: LACTIC ACIDOSIS
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin- associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metformin- associated lactic acidosis was characterized by elevated blood lactate levels (>5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally >5 mcg/mL [see Warnings and Precautions ( 5.1) ].
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g. carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high risk groups are provided [see Dosage and Administration ( 2.3), (2.7), Contraindications ( 4), Warnings and Precautions ( 5.1) ].
If metformin-associated lactic acidosis is suspected, immediately discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended [see Warnings and Precautions ( 5.1) ].
1 INDICATIONS AND USAGE
Metformin hydrochloride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults and pediatric patients 10 years of age and older with type 2 diabetes mellitus.
Metformin hydrochloride extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2 DOSAGE AND ADMINISTRATION
2.1 Adult Dosage
Metformin Hydrochloride Tablets
- The recommended starting dose of metformin hydrochloride tablets are 500 mg orally twice a day or 850 mg once a day, given with meals.
- Increase the dose in increments of 500 mg weekly or 850 mg every 2 weeks on the basis of glycemic control and tolerability, up to a maximum dose of 2,550 mg per day, given in divided doses.
- Doses above 2,000 mg may be better tolerated given 3 times a day with meals.
Metformin Hydrochloride Extended-Release Tablets
- Swallow metformin hydrochloride extended-release tablets whole and never crush, cut or chew.
- The recommended starting dose of metformin hydrochloride extended-release tablets are 500 mg orally once daily with the evening meal.
- Increase the dose in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2,000 mg once daily with the evening meal.
- If glycemic control is not achieved with metformin hydrochloride extended-release tablets 2,000 mg once daily, consider a trial of metformin hydrochloride extended-release tablets 1,000 mg twice daily. If higher doses are required, switch to metformin hydrochloride tablets at total daily doses up to 2,550 mg administered in divided daily doses, as described above.
- Patients receiving metformin hydrochloride tablets may be switched to metformin hydrochloride extended-release tablets once daily at the same total daily dose, up to 2,000 mg once daily.
2.2 Pediatric Dosage for Metformin Hydrochloride Tablets
- The recommended starting dose of metformin hydrochloride tablets for pediatric patients 10 years of age and older is 500 mg orally twice a day, given with meals.
- Increase dosage in increments of 500 mg weekly on the basis of glycemic control and tolerability, up to a maximum of 2,000 mg per day, given in divided doses twice daily.
2.3 Recommendations for Use in Renal Impairment
- Assess renal function prior to initiation of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and periodically thereafter.
- Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets are contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m 2.
- Initiation of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets in patients with an eGFR between 30 mL/minute/1.73 m 2to 45 mL/minute/1.73 m 2is not recommended.
- In patients taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets whose eGFR later falls below 45 mL/min/1.73 m 2, assess the benefit risk of continuing therapy.
- Discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if the patient's eGFR later falls below 30 mL/minute/1.73 m 2[see Warnings and Precautions ( 5.1) ].
2.4 Discontinuation for Iodinated Contrast Imaging Procedures
Discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m 2; in patients with a history of liver disease, alcoholism, or heart failure; or in patients who will be administered intra- arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if renal function is stable.
3 DOSAGE FORMS AND STRENGTHS
Metformin Hydrochloride Tablets, USP
- Metformin Hydrochloride Tablets USP, 500 mg are white to off-white, round shaped, film-coated tablets debossed with the logo of "70" on one side and "Z" on the other side.
- Metformin Hydrochloride Tablets USP, 850 mg are white to off-white, oval shaped, film-coated tablets debossed with the logo of "69" on one side and "Z" on the other side.
- Metformin Hydrochloride Tablets USP, 1,000 mg are white to off-white, oval shaped, biconvex, film-coated tablets with bisect on both the sides; one side of the bisect is debossed with "Z" and other side of the bisect is debossed with "71".
Metformin Hydrochloride Extended-release Tablets, USP
- Metformin Hydrochloride Extended-release Tablets USP, 500 mg are white to off-white, capsule shaped, uncoated tablets, debossed with "63" on one side and "Z" on the other side.
- Metformin Hydrochloride Extended-release Tablets USP, 750 mg are white to off-white, capsule shaped, uncoated tablets, debossed with "Z", "C" on one side and "20" on the other side.
4 CONTRAINDICATIONS
Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets are contraindicated in patients with:
- Severe renal impairment (eGFR below 30 mL/min/1.73 m 2) [see Warnings and Precautions ( 5.1) ].
- Hypersensitivity to metformin.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis
There have been postmarketing cases of metformin-associated lactic acidosis, including fatal cases. These cases had a subtle onset and were accompanied by nonspecific symptoms such as malaise, myalgias, abdominal pain, respiratory distress, or increased somnolence; however, hypotension and resistant bradyarrhythmias have occurred with severe acidosis. Metformin- associated lactic acidosis was characterized by elevated blood lactate concentrations (>5 mmol/L), anion gap acidosis (without evidence of ketonuria or ketonemia), and an increased lactate: pyruvate ratio; metformin plasma levels were generally >5 mcg/mL. Metformin decreases liver uptake of lactate increasing lactate blood levels which may increase the risk of lactic acidosis, especially in patients at risk.
If metformin-associated lactic acidosis is suspected, general supportive measures should be instituted promptly in a hospital setting, along with immediate discontinuation of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets. In metformin hydrochloride tablets or metformin hydrochloride extended-release tablets treated patients with a diagnosis or strong suspicion of lactic acidosis, prompt hemodialysis is recommended to correct the acidosis and remove accumulated metformin (metformin hydrochloride is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions). Hemodialysis has often resulted in reversal of symptoms and recovery.
Educate patients and their families about the symptoms of lactic acidosis and, if these symptoms occur, instruct them to discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and report these symptoms to their healthcare provider.
For each of the known and possible risk factors for metformin-associated lactic acidosis, recommendations to reduce the risk of and manage metformin-associated lactic acidosis are provided below:
-
Renal impairment: The postmarketing metformin-associated lactic acidosis cases primarily occurred in patients with significant renal impairment. The risk of metformin accumulation and metformin-associated lactic acidosis increases with the severity of renal impairment because metformin is substantially excreted by the kidney. Clinical recommendations based upon the patient's renal function include [see
Dosage and Administration (
2.1), Clinical Pharmacology (
12.3)
]:
- Before initiating metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, obtain an estimated glomerular filtration rate (eGFR).
- Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets are contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2 [see Contraindications ( 4)].
- Initiation of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets is not recommended in patients with eGFR between 30 mL/min/1.73 m2 to 45 mL/min/1.73 m2.
- Obtain an eGFR at least annually in all patients taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets. In patients at risk for the development of renal impairment (e.g., the elderly), renal function should be assessed more frequently.
- In patients taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets whose eGFR falls below 45 mL/min/1.73 m2, assess the benefit and risk of continuing therapy.
- Drug interactions : The concomitant use of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets with specific drugs may increase the risk of metformin-associated lactic acidosis: those that impair renal function, result in significant hemodynamic change, interfere with acid-base balance, or increase metformin accumulation. Consider more frequent monitoring of patients.
- Age 65 or greater: The risk of metformin-associated lactic acidosis increases with the patient's age because elderly patients have a greater likelihood of having hepatic, renal, or cardiac impairment than younger patients. Assess renal function more frequently in elderly patients.
- Radiologic studies with contrast : Administration of intravascular iodinated contrast agents in metformin-treated patients has led to an acute decrease in renal function and the occurrence of lactic acidosis. Stop metformin hydrochloride tablets or metformin hydrochloride extended-release tablets at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 mL/min/1.73 m 2and 60 mL/min/1.73 m 2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure, and restart metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if renal function is stable.
- Surgery and other procedures: Withholding of food and fluids during surgical or other procedures may increase the risk for volume depletion, hypotension, and renal impairment. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets should be temporarily discontinued while patients have restricted food and fluid intake.
- Hypoxic states: Several of the postmarketing cases of metformin-associated lactic acidosis occurred in the setting of acute congestive heart failure (particularly when accompanied by hypoperfusion and hypoxemia). Cardiovascular collapse (shock), acute myocardial infarction, sepsis, and other conditions associated with hypoxemia have been associated with lactic acidosis and may cause prerenal azotemia. When such an event occurs, discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
- Excessive alcohol intake: Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
- Hepatic impairment: Patients with hepatic impairment have developed cases of metformin-associated lactic acidosis. This may be due to impaired lactate clearance resulting in higher lactate blood levels. Therefore, avoid use of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets in patients with clinical or laboratory evidence of hepatic disease.
5.2 Vitamin B12 Deficiency
In metformin hydrochloride tablets clinical trials of 29 week duration, a decrease to subnormal levels of previously normal serum vitamin B 12 levels was observed in approximately 7% of patients. Such decrease, possibly due to interference with B 12 absorption from the B 12-intrinsic factor complex, may be associated with anemia but appears to be rapidly reversible with discontinuation of Metformin hydrochloride tablets or vitamin B 12 supplementation. Certain individuals (those with inadequate vitamin B 12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B 12 levels. Measure hematologic parameters on an annual basis and vitamin B 12 at 2 to 3 year intervals in patients on metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and manage any abnormalities [see Adverse Reactions ( 6.1) ].
5.3 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Insulin and insulin secretagogues (e.g., sulfonylurea) are known to cause hypoglycemia. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets may increase the risk of hypoglycemia when combined with insulin and/or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets [see Drug Interactions ( 7)].
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere in the labeling:
- Lactic Acidosis [see Boxed Warning and Warnings and Precautions ( 5.1)]
- Vitamin B 12Deficiency [see Warnings and Precautions ( 5.2)]
- Hypoglycemia [see Warnings and Precautions ( 5.3)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Metformin Hydrochloride Tablets
In a U.S. clinical trial of metformin hydrochloride tablets in patients with type 2 diabetes mellitus, a total of 141 patients received metformin hydrochloride tablets up to 2,550 mg per day. Adverse reactions reported in greater than 5% of metformin hydrochloride tablets treated patients and that were more common than in placebo-treated patients, are listed in Table 1.
| Metformin Hydrochloride Tablets
| Placebo
|
|
| (n=141)
| (n=145)
|
|
| Diarrhea
| 53%
| 12%
|
| Nausea/Vomiting
| 26%
| 8%
|
| Flatulence
| 12%
| 6%
|
| Asthenia
| 9%
| 6%
|
| Indigestion
| 7%
| 4%
|
| Abdominal Discomfort
| 6%
| 5%
|
| Headache
| 6%
| 5%
|
Diarrhea led to discontinuation of metformin hydrochloride tablets in 6% of patients. Additionally, the following adverse reactions were reported in ≥1% to ≤5% of metformin hydrochloride tablets treated patients and were more commonly reported with metformin hydrochloride tablets than placebo: abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating increased, taste disorder, chest discomfort, chills, flu syndrome, flushing, palpitation.
In metformin hydrochloride tablets clinical trials of 29 week duration, a decrease to subnormal levels of previously normal serum vitamin B 12 levels was observed in approximately 7% of patients.
Pediatric Patients
In clinical trials with metformin hydrochloride tablets in pediatric patients with type 2 diabetes mellitus, the profile of adverse reactions was similar to that observed in adults.
Metformin Hydrochloride Extended-Release Tablets
In placebo-controlled trials, 781 patients were administered metformin hydrochloride extended-release tablets. Adverse reactions reported in greater than 5% of the metformin hydrochloride extended-release tablets patients, and that were more common in metformin hydrochloride extended-release tablets- than placebo-treated patients, are listed in Table 2.
| Metformin Hydrochloride Extended-Release Tablets
| Placebo
|
|
| (n=781)
| (n=195)
|
|
| Diarrhea
| 10%
| 3%
|
| Nausea/Vomiting
| 7%
| 2%
|
Diarrhea led to discontinuation of metformin hydrochloride extended-release tablets in 0.6% of patients. Additionally, the following adverse reactions were reported in ≥1.0% to ≤5.0% of metformin hydrochloride extended-release tablets patients and were more commonly reported with metformin hydrochloride extended-release tablets than placebo: abdominal pain, constipation, distention abdomen, dyspepsia/heartburn, flatulence, dizziness, headache, upper respiratory infection, taste disturbance.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of metformin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cholestatic, hepatocellular, and mixed hepatocellular liver injury have been reported with postmarketing use of metformin.
7 DRUG INTERACTIONS
Table 3 presents clinically significant drug interactions with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
| Carbonic Anhydrase Inhibitors
|
|
| Clinical Impact:
| Carbonic anhydrase inhibitors frequently cause a decrease in serum bicarbonate and induce non-anion gap, hyperchloremic metabolic acidosis. Concomitant use of these drugs with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets may increase the risk for lactic acidosis.
|
| Intervention:
| Consider more frequent monitoring of these patients.
|
| Examples:
| Topiramate, zonisamide, acetazolamide or dichlorphenamide.
|
| Drugs that Reduce Metformin Hydrochloride Tablets or metformin Hydrochloride Extended-Release Tablets Clearance
|
|
| Clinical Impact:
| Concomitant use of drugs that interfere with common renal tubular transport systems involved in the renal elimination of metformin (e.g., organic cationic transporter-2 [OCT2] / multidrug and toxin extrusion [MATE] inhibitors) could increase systemic exposure to metformin and may increase the risk for lactic acidosis
[see Clinical Pharmacology (
12.3)].
|
| Intervention:
| Consider the benefits and risks of concomitant use with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
|
| Examples:
| Ranolazine, vandetanib, dolutegravir, and cimetidine.
|
| Alcohol
|
|
| Clinical Impact:
| Alcohol is known to potentiate the effect of metformin on lactate metabolism.
|
| Intervention:
| Warn patients against excessive alcohol intake while receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
|
| Insulin Secretagogues or Insulin
|
|
| Clinical Impact:
| Coadministration of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia.
|
| Intervention:
| Patients receiving an insulin secretagogue or insulin may require lower doses of the insulin secretagogue or insulin.
|
| Drugs Affecting Glycemic Control
|
|
| Clinical Impact:
| Certain drugs tend to produce hyperglycemia and may lead to loss of glycemic control.
|
| Intervention:
| When such drugs are administered to a patient receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, observe the patient closely for loss of blood glucose control. When such drugs are withdrawn from a patient receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, observe the patient closely for hypoglycemia.
|
| Examples:
| Thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blockers, and isoniazid.
|
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Limited data with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. Published studies with metformin use during pregnancy have not reported a clear association with metformin and major birth defect or miscarriage risk [see Data]. There are risks to the mother and fetus associated with poorly controlled diabetes mellitus in pregnancy [see Clinical Considerations].
No adverse developmental effects were observed when metformin was administered to pregnant Sprague Dawley rats and rabbits during the period of organogenesis at doses up to 2 times and 5 times, respectively, a 2,550 mg clinical dose, based on body surface area [see Data].
The estimated background risk of major birth defects is 6% to 10% in women with pre-gestational diabetes mellitus with an HbA1C >7 and has been reported to be as high as 20% to 25% in women with a HbA1C >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly-controlled diabetes mellitus in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth and delivery complications. Poorly controlled diabetes mellitus increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from postmarketing studies have not reported a clear association with metformin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when metformin was used during pregnancy. However, these studies cannot definitely establish the absence of any metformin-associated risk because of methodological limitations, including small sample size and inconsistent comparator groups.
Animal Data
Metformin hydrochloride did not adversely affect development outcomes when administered to pregnant rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 times and 5 times a 2,550 mg clinical dose based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.2 Lactation
Limited published studies report that metformin is present in human milk [see Data]. However, there is insufficient information to determine the effects of metformin on the breastfed infant and no available information on the effects of metformin on milk production. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and any potential adverse effects on the breastfed child from metformin hydrochloride tablets or metformin hydrochloride extended-release tablets or from the underlying maternal condition.
Data
Published clinical lactation studies report that metformin is present in human milk which resulted in infant doses approximately 0.11% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.13 and 1. However, the studies were not designed to definitely establish the risk of use of metformin during lactation because of small sample size and limited adverse event data collected in infants.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Metformin Hydrochloride Tablets
The safety and effectiveness of metformin hydrochloride tablets for the treatment of type 2 diabetes mellitus have been established in pediatric patients 10 years to 16 years old. Safety and effectiveness of metformin hydrochloride tablets have not been established in pediatric patients less than 10 years old.
Use of metformin hydrochloride tablets in pediatric patients 10 years to 16 years old for the treatment of type 2 diabetes mellitus is supported by evidence from adequate and well-controlled studies of metformin hydrochloride tablets in adults with additional data from a controlled clinical study in pediatric patients 10 years to 16 years old with type 2 diabetes mellitus, which demonstrated a similar response in glycemic control to that seen in adults [see Clinical Studies (14.1)]. In this study, adverse reactions were similar to those described in adults. A maximum daily dose of 2,000 mg of metformin hydrochloride tablets are recommended. [See Dosage and Administration (2.2).]
Metformin Hydrochloride Extended-Release Tablets
Safety and effectiveness of metformin hydrochloride extended-release tablets in pediatric patients have not been established.
8.5 Geriatric Use
Controlled clinical studies of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of lactic acidosis. Assess renal function more frequently in elderly patients [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Metformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of renal impairment. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets are contraindicated in severe renal impairment, patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m 2 [see Dosage and Administration (2.3), Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Use of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets are not recommended in patients with hepatic impairment. [see Warnings and Precautions ( 5.1) ].
10 OVERDOSAGE
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions ( 5.1) ]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
11 DESCRIPTION
Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin hydrochloride is N,N-dimethylimidodicarbonimidic diamide hydrochloride. The structural formula is as shown below:
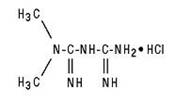
Metformin hydrochloride, USP is a white crystalline powder with a molecular formula of C 4H 11N 5 HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol and is practically insoluble in acetone and methylene chloride. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68.
Metformin hydrochloride tablets, USP contain 500 mg or 850 mg or 1,000 mg of metformin hydrochloride, USP. Each tablet contains the inactive ingredients colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone and sodium starch glycolate.
Metformin hydrochloride extended-release tablets, USP contain 500 mg or 750 mg of metformin hydrochloride, USP as the active ingredient.
Metformin hydrochloride extended-release tablets, USP 500 mg and 750 mg contain the inactive ingredients glyceryl behenate, hypromellose, microcrystalline cellulose and povidone.
The methodology used for dissolution testing is USP Test 1 for metformin hydrochloride tablets, USP.
The methodology used for dissolution testing is USP Test 8 for metformin hydrochloride extended-release tablets, USP.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metformin is an antihyperglycemic agent which improves glucose tolerance in patients with type 2 diabetes mellitus, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may decrease.
12.3 Pharmacokinetics
The absolute bioavailability of metformin hydrochloride tablets, 500 mg given under fasting conditions is approximately 50% to 60%. Studies using single oral doses of metformin hydrochloride tablets 500 mg to 1,500 mg and 850 mg to 2,550 mg, indicate that there is a lack of dose proportionality with increasing doses, which is due to decreased absorption rather than an alteration in elimination. At usual clinical doses and dosing schedules of metformin hydrochloride tablets, steady state plasma concentrations of metformin are reached within 24 hours to 48 hours and are generally <1 μg/mL.
Following a single oral dose of metformin hydrochloride extended-release tablets, C max is achieved with a median value of 7 hours and a range of 4 hours to 8 hours. Peak plasma levels are approximately 20% lower compared to the same dose of metformin hydrochloride tablets, however, the extent of absorption (as measured by AUC) is comparable to metformin hydrochloride tablets.
At steady state, the AUC and C max are less than dose proportional for metformin hydrochloride extended-release tablets within the range of 500 to 2,000 mg administered once daily. Peak plasma levels are approximately 0.6 mcg/mL, 1.1 mcg/mL, 1.4 mcg/mL and 1.8 mcg/mL for 500 mg, 1,000 mg, 1,500 mg and 2,000 mg once-daily doses, respectively. The extent of metformin absorption (as measured by AUC) from metformin hydrochloride extended-release tablets at a 2,000 mg once-daily dose is similar to the same total daily dose administered as metformin hydrochloride tablets 1,000 mg twice daily. After repeated administration of metformin hydrochloride extended-release tablets, metformin did not accumulate in plasma.
Effect of food:
Food decreases the extent of absorption and slightly delays the absorption of metformin, as shown by approximately a 40% lower mean peak plasma concentration (C max), a 25% lower area under the plasma concentration versus time curve (AUC), and a 35-minute prolongation of time to peak plasma concentration (T max) following administration of a single 850 mg tablet of metformin hydrochloride tablets with food, compared to the same tablet strength administered fasting.
Although the extent of metformin absorption (as measured by AUC) from the metformin hydrochloride extended-release tablets tablet increased by approximately 50% when given with food, there was no effect of food on C max and T max of metformin. Both high and low fat meals had the same effect on the pharmacokinetics of metformin hydrochloride extended-release tablets.
Distribution
The apparent volume of distribution (V/F) of metformin following single oral doses of metformin hydrochloride tablets 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins. Metformin partitions into erythrocytes, most likely as a function of time.
Metabolism
Intravenous single-dose studies in normal subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) nor biliary excretion.
Elimination
Renal clearance (see Table 4) is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
In patients with decreased renal function the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased (see Table 3) [See Dosage and Administration ( 2.3), Contraindications ( 4), Warnings and Precautions ( 5.1) and Use in Specific Populations ( 8.6) ].
Hepatic Impairment
No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment [See Warnings and Precautions ( 5.1) and Use in Specific Populations ( 8.7) ] .
Geriatrics
Limited data from controlled pharmacokinetic studies of metformin hydrochloride extended-release tablets in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and C max is increased, compared to healthy young subjects. It appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function (see Table 4). [See Warnings and Precautions ( 5.1) and Use in Specific Populations ( 8.5) ].
|
a All doses given fasting except the first 18 doses of the multiple dose studies |
|||
|
b Peak plasma concentration |
|||
|
c Time to peak plasma concentration |
|||
|
d Combined results (average means) of five studies: mean age 32 years (range 23 years to59 years) |
|||
|
e Kinetic study done following dose 19, given fasting |
|||
|
f Elderly subjects, mean age 71 years (range 65 years to 81 years) |
|||
|
g CL cr= creatinine clearance normalized to body surface area of 1.73 m 2 |
|||
| Subject Groups: Metformin Hydrochloride Tablets dose
a
(number of subjects) | Cmaxb
(mcg/mL) | Tmaxc
(hrs) | Renal Clearance
(mL/min) |
| Healthy, nondiabetic adults:
500 mg single dose (24) 850 mg single dose (74) d 850 mg three times daily for 19 doses e (9) | 1.03 (±0.33)
1.60 (±0.38) 2.01 (±0.42) | 2.75 (±0.81)
2.64 (±0.82) 1.79 (±0.94) | 600 (±132)
552 (±139) 642 (±173) |
| Adults with type 2 diabetes mellitus:
850 mg single dose (23) 850 mg three times daily for 19 doses e (9) | 1.48 (±0.5)
1.90 (±0.62) | 3.32 (±1.08)
2.01 (±1.22) | 491 (±138)
550 (±160) |
| Elderlyf , healthy nondiabetic adults:
850 mg single dose (12) | 2.45 (±0.70)
| 2.71 (±1.05)
| 412 (±98)
|
| Renal-impaired adults:
850 mg single dose Mild (CL crg61 mL/min to 90 mL/min) (5) Moderate (CL cr 31 mL/min to 60 mL/min) (4) Severe (CL cr 10 mL/min to 30 mL/min) (6) | 1.86 (±0.52)
4.12 (±1.83) 3.93 (±0.92) | 3.20 (±0.45)
3.75 (±0.50) 4.01 (±1.10) | 384 (±122)
108 (±57) 130 (±90) |
After administration of a single oral metformin hydrochloride tablets 500 mg tablet with food, geometric mean metformin C max and AUC differed less than 5% between pediatric type 2 diabetic patients (12 years to 16 years of age) and gender- and weight-matched healthy adults (20 years to 45 years of age), all with normal renal function.
Gender
Metformin pharmacokinetic parameters did not differ significantly between normal subjects and patients with type 2 diabetes mellitus when analyzed according to gender (males=19, females=16).
Race
No studies of metformin pharmacokinetic parameters according to race have been performed.
Drug Interactions
In Vivo Assessment of Drug Interactions
|
* All metformin and coadministered drugs were given as single doses |
|||||
|
† AUC = AUC(INF) |
|||||
|
‡ Ratio of arithmetic means |
|||||
|
§ At steady state with topiramate 100 mg every 12 hours and metformin 500 mg every 12 hours; AUC = AUC 0 to 12h |
|||||
| Coadministered
Drug | Dose of Coadministered
Drug * | Dose of
Metformin * | Geometric Mean Ratio
(ratio with/without coadministered drug) No Effect = 1.00 |
||
| AUC†
| Cmax
|
||||
| No dosing adjustments required for the following:
|
|||||
| Glyburide
| 5 mg
| 850 mg
| metformin
| 0.91
‡
| 0.93
‡
|
| Furosemide
| 40 mg
| 850 mg
| metformin
| 1.09
‡
| 1.22
‡
|
| Nifedipine
| 10 mg
| 850 mg
| metformin
| 1.16
| 1.21
|
| Propranolol
| 40 mg
| 850 mg
| metformin
| 0.90
| 0.94
|
| Ibuprofen
| 400 mg
| 850 mg
| metformin
| 1.05
‡
| 1.07
‡
|
| Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination [See
Warnings and Precautions (5.9) and
Drug Interactions (7.2). ]
|
|||||
| Cimetidine
| 400 mg
| 850 mg
| metformin
| 1.40
| 1.61
|
| Carbonic anhydrase inhibitors may cause metabolic acidosis [See
Warnings and Precautions (5.1) and
Drug Interactions (7.1). ]
|
|||||
| Topiramate
| 100 mg
§
| 500 mg
§
| metformin
| 1.25
§
| 1.17
|
|
* All metformin and coadministered drugs were given as single doses |
|||||
|
† AUC = AUC(INF) unless otherwise noted |
|||||
|
‡ Ratio of arithmetic means, p-value of difference <0.05 |
|||||
|
§ AUC(0hr to 24 hr) reported |
|||||
|
¶ Ratio of arithmetic means |
|||||
| Coadministered
Drug | Dose of
Coadministered Drug* | Dose of
Metformin* | Geometric Mean Ratio (ratio with/without metformin)
No Effect = 1 |
||
| AUC†
| C
max
|
||||
| No dosing adjustments required for the following:
|
|||||
| Glyburide
| 5 mg
| 850 mg
| glyburide
| 0.78
‡
| 0.63
‡
|
| Furosemide
| 40 mg
| 850 mg
| furosemide
| 0.87
‡
| 0.69
‡
|
| Nifedipine
| 10 mg
| 850 mg
| nifedipine
| 1.10
§
| 1.08
|
| Propranolol
| 40 mg
| 850 mg
| propranolol
| 1.01
§
| 1.02
|
| Ibuprofen
| 400 mg
| 850 mg
| ibuprofen
| 0.97
¶
| 1.01
¶
|
| Cimetidine
| 400 mg
| 850 mg
| cimetidine
| 0.95
§
| 1.01
|
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1,500 mg/kg/day, respectively. These doses are both approximately 3 times the maximum recommended human daily dose of 2,550 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test ( S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 2 times the maximum recommended human daily dose of 2,550 mg based on body surface area comparisons.
14 CLINICAL STUDIES
14.1 Metformin Hydrochloride Tablets
A double-blind, placebo-controlled, multi-center US clinical trial involving obese patients with type 2 diabetes mellitus whose hyperglycemia was not adequately controlled with dietary management alone (baseline fasting plasma glucose [FPG] of approximately 240 mg/dL) was conducted. Patients were treated with metformin hydrochloride (up to 2,550 mg/day) or placebo for 29 weeks. The results are presented in Table 7.
|
* Not statistically significant |
|||
| Metformin Hydrochloride Tablets
(n=141) | Placebo
(n=145) | p-Value
|
|
| FPG (mg/dL)
Baseline Change at FINAL VISIT | 241.5
–53.0 | 237.7
6.3 | NS
*
0.001 |
| Hemoglobin A1c (%)
Baseline Change at FINAL VISIT | 8.4
–1.4 | 8.2
0.4 | NS
*
0.001 |
Mean baseline body weight was 201 lbs and 206 lbs in the metformin hydrochloride and placebo arms, respectively. Mean change in body weight from baseline to week 29 was -1.4 lbs and -2.4 lbs in the metformin hydrochloride and placebo arms, respectively. A 29 week, double-blind, placebo-controlled study of metformin hydrochloride and glyburide, alone and in combination, was conducted in obese patients with type 2 diabetes mellitus who had failed to achieve adequate glycemic control while on maximum doses of glyburide (baseline FPG of approximately 250 mg/dL). Patients randomized to the combination arm started therapy with metformin hydrochloride 500 mg and glyburide 20 mg. At the end of each week of the first 4 weeks of the trial, these patients had their dosages of metformin hydrochloride increased by 500 mg if they had failed to reach target fasting plasma glucose. After week 4, such dosage adjustments were made monthly, although no patient was allowed to exceed metformin hydrochloride 2,500 mg. Patients in the metformin hydrochloride only arm (metformin plus placebo) discontinued glyburide and followed the same titration schedule. Patients in the glyburide arm continued the same dose of glyburide. At the end of the trial, approximately 70% of the patients in the combination group were taking metformin hydrochloride 2,000 mg/glyburide 20 mg or metformin hydrochloride 2,500 mg/glyburide 20 mg. The results are displayed in Table 8.
|
* Not statistically significant |
||||||
| Comb
(n=213) | Glyb
(n=209) | GLU (n=210)
| p-Values
|
|||
| Glyb vs. Comb
| GLU vs. Comb
| GLU vs. Glyb
|
||||
| Fasting Plasma Glucose (mg/dL)
Baseline Change at FINAL VISIT | 250.5
–63.5 | 247.5
13.7 | 253.9
–0.9 | NS
*
0.001 | NS
*
0.001 | NS
*
0.025 |
| Hemoglobin A1c (%)
Baseline Change at FINAL VISIT | 8.8
–1.7 | 8.5
0.2 | 8.9
–0.4 | NS
*
0.001 | NS
*
0.001 | 0.007
0.001 |
Mean baseline body weight was 202 lbs, 203 lbs, and 204 lbs in the metformin hydrochloride/glyburide, glyburide, and metformin hydrochloride arms, respectively. Mean change in body weight from baseline to week 29 was 0.9 lbs, -0.7 lbs, and -8.4 lbs in the metformin hydrochloride/glyburide, glyburide, and metformin hydrochloride arms, respectively.
Pediatric Clinical Studies
A double-blind, placebo-controlled study in pediatric patients aged 10 to 16 years with type 2 diabetes mellitus (mean FPG 182.2 mg/dL), treatment with metformin hydrochloride (up to 2,000 mg/day) for up to 16 weeks (mean duration of treatment 11 weeks) was conducted. The results are displayed in Table 9.
|
a Pediatric patients mean age 13.8 years (range 10 years to 16 years) |
|||
| Metformin Hydrochloride
| Placebo
| p-Value
|
|
| FPG (mg/dL)
| (n=37)
| (n=36)
| |
| Baseline
| 162.4
| 192.3
| |
| Change at FINAL VISIT
| –42.9
| 21.4
| <0.001
|
Mean baseline body weight was 205 lbs and 189 lbs in the metformin hydrochloride and placebo arms, respectively. Mean change in body weight from baseline to week 16 was -3.3 lbs and -2.0 lbs in the metformin hydrochloride and placebo arms, respectively.
14.2 Metformin Hydrochloride Extended-Release Tablets
A 24 week, double-blind, placebo-controlled study of metformin hydrochloride extended-release tablets, taken once daily with the evening meal, was conducted in patients with type 2 diabetes mellitus who had failed to achieve glycemic control with diet and exercise. Patients entering the study had a mean baseline HbA1c of 8.0% and a mean baseline FPG of 176 mg/dL. The treatment dose was increased to 1,500 mg once daily if at Week 12 HbA1c was ≥7.0% but <8.0% (patients with HbA1c ≥8.0% were discontinued from the study). At the final visit (24 week), mean HbA1c had increased 0.2% from baseline in placebo patients and decreased 0.6% with metformin hydrochloride extended-release tablets.
A 16 week, double-blind, placebo-controlled, dose-response study of metformin hydrochloride extended-release tablets, taken once daily with the evening meal or twice daily with meals, was conducted in patients with type 2 diabetes mellitus who had failed to achieve glycemic control with diet and exercise. The results are shown in Table 10.
|
a All comparisons versus Placebo |
||||||
| Metformin Hydrochloride Extended-Release Tablets
| Placebo
|
|||||
| 500 mg Once Daily
| 1,000 mg Once Daily
| 1,500 mg Once Daily
| 2,000 mg Once Daily
| 1,000 mg Twice Daily
|
||
| Hemoglobin A1c (%)
| (n=115)
| (n=115)
| (n=111)
| (n=125)
| (n=112)
| (n=111)
|
| Baseline
| 8.2
| 8.4
| 8.3
| 8.4
| 8.4
| 8.4
|
| Change at FINAL VISIT
| –0.4
| –0.6
| –0.9
| –0.8
| –1.1
| 0.1
|
| p-value
a
| <0.001
| <0.001
| <0.001
| <0.001
| <0.001
| –
|
| FPG (mg/dL)
| (n=126)
| (n=118)
| (n=120)
| (n=132)
| (n=122)
| (n=113)
|
| Baseline
| 182.7
| 183.7
| 178.9
| 181.0
| 181.6
| 179.6
|
| Change at FINAL VISIT
| –15.2
| –19.3
| –28.5
| –29.9
| –33.6
| 7.6
|
| p-value
a
| <0.001
| <0.001
| <0.001
| <0.001
| <0.001
| –
|
Mean baseline body weight was 193 lbs, 192 lbs, 188 lbs, 196 lbs, 193 lbs and 194 lbs in the metformin hydrochloride extended-release tablets 500 mg , 1,000 mg, 1,500 mg, and 2,000 mg once daily, 1,000 mg twice daily and placebo arms, respectively. Mean change in body weight from baseline to week 16 was -1.3 lbs, -1.3 lbs, -0.7 lbs, -1.5 lbs, -2.2 lbs and -1.8 lbs, respectively.
A 24 week, double-blind, randomized study of metformin hydrochloride extended-release tablets, taken once daily with the evening meal, and metformin hydrochloride tablets, taken twice daily (with breakfast and evening meal), was conducted in patients with type 2 diabetes mellitus who had been treated with metformin hydrochloride tablets 500 mg twice daily for at least 8 weeks prior to study entry. The results are shown in Table 11.
|
*a n=68 |
|||
| Metformin Hydrochloride Tablets
500 mg Twice Daily | Metformin Hydrochloride Extended-Release Tablets
|
||
| 1,000 mg
Once Daily | 1,500 mg
Once Daily |
||
| Hemoglobin A1c (%)
Baseline Change at FINAL VISIT (95% CI) | (n=67)
7.06 0.14 a (–0.04, 0.31) | (n=72)
6.99 0.27 (0.11, 0.43) | (n=66)
7.02 0.13 (–0.02, 0.28) |
| FPG (mg/dL)
Baseline Change at FINAL VISIT (95% CI) | (n=69)
127.2 14.0 (7.0, 21.0) | (n=72)
131.0 11.5 (4.4, 18.6) | (n=70)
131.4 7.6 (1.0, 14.2) |
Mean baseline body weight was 210 lbs, 203 lbs and 193 lbs in the metformin hydrochloride tablets 500 mg twice daily, and metformin hydrochloride extended-release tablets 1,000 mg and 1,500 mg once daily arms, respectively. Mean change in body weight from baseline to week 24 was 0.9 lbs, 1.1 lbs and 0.9 lbs, respectively.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Metformin Hydrochloride Tablets, USP
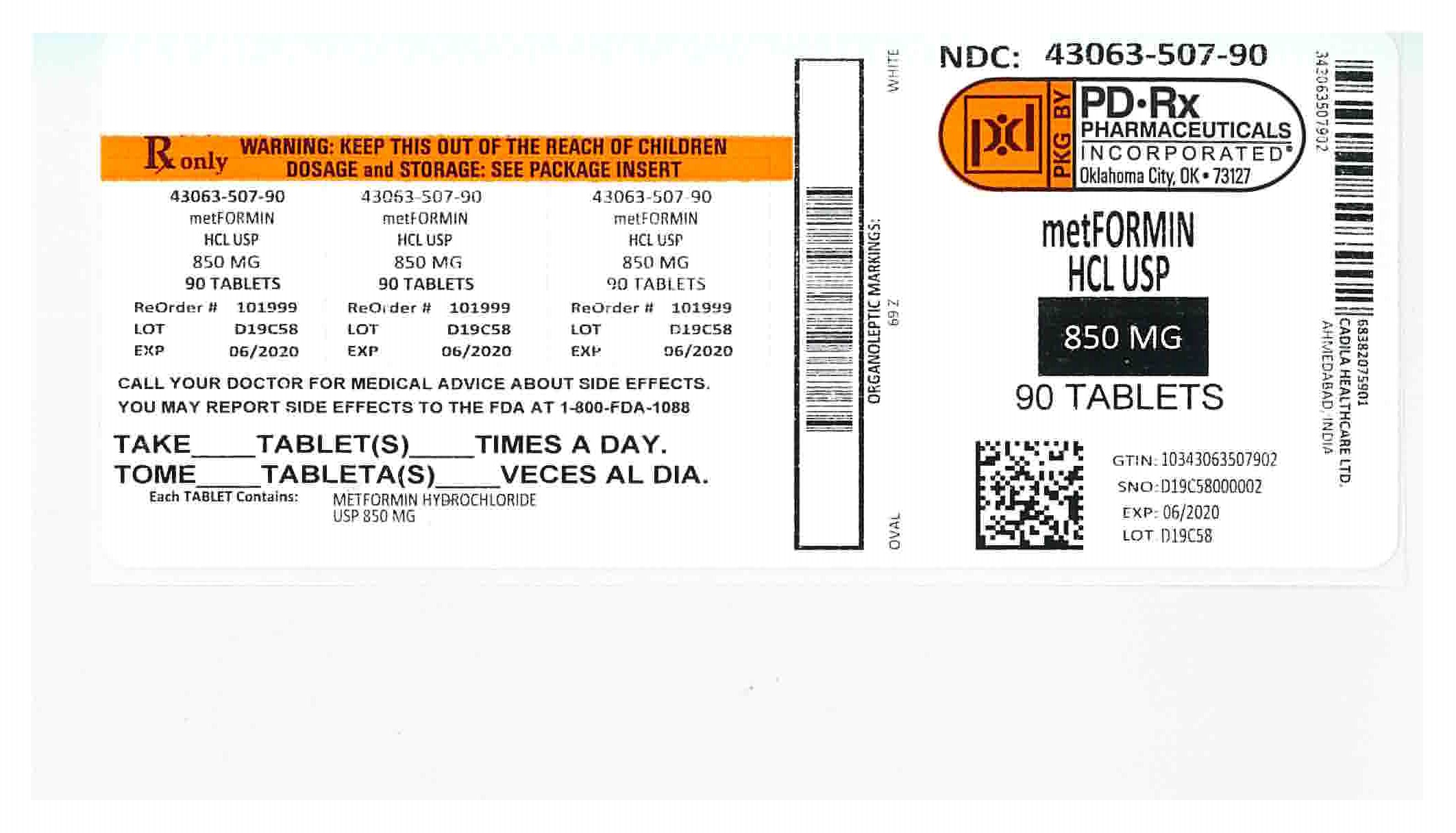
Metformin Hydrochloride Tablets USP, 850 mg are white to off-white, oval shaped, film-coated tablets debossed with the logo of "69" on one side and "Z" on the other side and are supplied as follows.
NDC: 43063-507-30 in bottles of 30 tablets
NDC: 43063-507-60 in bottles of 60 tablets
NDC: 43063-507-90 in bottles of 90 tablets
NDC: 43063-507-98 in bottles of 120 tablets
NDC: 43063-507-93 in bottles of 180 tablets
NDC: 43063-507-94 in bottles of 270 tablets
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Lactic Acidosis
Explain the risks of lactic acidosis, its symptoms, and conditions that predispose to its development. Advise patients to discontinue metformin hydrochloride tablets or metformin hydrochloride extended-release tablets immediately and to promptly notify their healthcare provider if unexplained hyperventilation, myalgias, malaise, unusual somnolence or other nonspecific symptoms occur. Counsel patients against excessive alcohol intake and inform patients about importance of regular testing of renal function while receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets. Instruct patients to inform their doctor that they are taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets prior to any surgical or radiological procedure, as temporary discontinuation may be required [see Warnings and Precautions ( 5.1)].
Hypoglycemia
Inform patients that hypoglycemia may occur when metformin hydrochloride tablets or metformin hydrochloride extended-release tablets are coadministered with oral sulfonylureas and insulin. Explain to patients receiving concomitant therapy the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development [see Warnings and Precautions ( 5.3)].
Vitamin B 12 Deficiency
Inform patients about importance of regular hematological parameters while receiving metformin hydrochloride tablets or metformin hydrochloride extended-release tablets [see Warnings and Precautions ( 5.2)] .
Females of Reproductive Age
Inform females that treatment with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets may result in ovulation in some premenopausal anovulatory women which may lead to unintended pregnancy [see Use in Specific Populations ( 8.3)].
Metformin hydrochloride extended-release tablets Administration Information
Inform patients that metformin hydrochloride extended-release tablets must be swallowed whole and not crushed, cut, or chewed, and that the inactive ingredients may occasionally be eliminated in the feces as a soft mass that may resemble the original tablet.
Cadila Healthcare Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 07/18
Metformin Hydrochloride (met for' min hye'' droe klor' ide) Tablets, USP
Metformin Hydrochloride (met for' min hye'' droe klor' ide) Extended-release Tablets, USP
Read the Patient Information that comes with metformin hydrochloride tablets and metformin hydrochloride extended-release tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Serious side effects can happen in people taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, including:
Lactic Acidosis. Metformin hydrochloride, the medicine in metformin hydrochloride tablets and metformin hydrochloride extended-release tablets, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
- feel very weak and tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unusual sleepiness or sleep longer than usual
- have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis if you:
- have kidney problems. People whose kidneys are not working properly should not take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
- have liver problems.
- have congestive heart failure that requires treatment with medicines.
- drink a lot of alcohol (very often or short-term "binge" drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery.
- have a heart attack, severe infection, or stroke.
- are 80 years of age or older and have not had your kidney function tested.
What are metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
- Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets are prescription medicines that contain metformin hydrochloride. Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets are used with diet and exercise to help control high blood sugar (hyperglycemia) in adults with type 2 diabetes.
- Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets are not for people with type 1 diabetes.
- Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets are not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets have the same active ingredient. However, metformin hydrochloride extended-release tablets works longer in your body. Both of these medicines help control your blood sugar in a number of ways. These include helping your body respond better to the insulin it makes naturally, decreasing the amount of sugar your liver makes, and decreasing the amount of sugar your intestines absorb. Metformin hydrochloride tablets and metformin hydrochloride extended-release tablets do not cause your body to make more insulin.
Who should not take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
Some conditions increase your chance of getting lactic acidosis, or cause other problems if you take either of these medicines. Most of the conditions listed below can increase your chance of getting lactic acidosis.
Do not take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if you:
- have kidney problems
- are allergic to the metformin hydrochloride in metformin hydrochloride tablets or metformin hydrochloride extended-release tablets
- or any of the ingredients in metformin hydrochloride tablets or metformin hydrochloride extended-release tablets. See the end of this leaflet for a complete list of ingredients in metformin hydrochloride tablets and metformin hydrochloride extended-release tablets.
- are going to get an injection of dye or contrast agents for an x-ray procedure or if you are going to have surgery and not able to eat or drink much. In these situations, metformin hydrochloride tablets or metformin hydrochloride extended-release tablets will need to be stopped for a short time. Talk to your healthcare provider about when you should stop metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and when you should start metformin hydrochloride tablets or metformin hydrochloride extended-release tablets again. See " What is the most important information I should know about metformin hydrochloride tablets or metformin hydrochloride extended-release tablets? "
What should I tell my healthcare provider before taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
Before taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, tell your healthcare provider if you:
- have type 1 diabetes. Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets should not be used to treat people with type 1 diabetes.
- have a history or risk for diabetic ketoacidosis (high levels of certain acids, known as ketones, in the blood or urine). Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets should not be used for the treatment of diabetic ketoacidosis.
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are older than 80 years. If you are over 80 years old you should not take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets unless your kidneys have been checked and they are normal.
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking.
- are taking insulin.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if metformin hydrochloride tablets or metformin hydrochloride extended-release tablets will harm your unborn baby. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are breast-feeding or plan to breast-feed. It is not known if metformin hydrochloride passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
- Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets may affect the way other medicines work, and other medicines may affect how metformin hydrochloride tablets or metformin hydrochloride extended-release tablets works.
Can metformin hydrochloride tablets or metformin hydrochloride extended-release tablets be used in children?
Metformin hydrochloride tablets have been shown to effectively lower glucose levels in children (ages 10 years to 16 years) with type 2 diabetes. Metformin hydrochloride tablets have not been studied in children younger than 10 years old. Metformin hydrochloride tablets have not been studied in combination with other oral glucose-control medicines or insulin in children. If you have any questions about the use of metformin hydrochloride tablets in children, talk with your doctor or other healthcare provider.
Metformin hydrochloride extended-release tablets have not been studied in children.
How should I take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
- Take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets exactly as your healthcare provider tells you.
- Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets should be taken with meals to help lessen an upset stomach side effect.
- Swallow metformin hydrochloride tablets or metformin hydrochloride extended-release tablets whole. Do not crush, cut, or chew metformin hydrochloride extended-release tablets.
- You may sometimes pass a soft mass in your stools (bowel movement) that looks like metformin hydrochloride extended-release tablets. This is not harmful and will not affect the way metformin hydrochloride extended-release tablets works to control your diabetes.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your healthcare provider right away if you have any of these problems.
- Your healthcare provider should do blood tests to check how well your kidneys are working before and during your treatment with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Follow your healthcare provider's instructions for treating blood sugar that is too low (hypoglycemia). Talk to your healthcare provider if low blood sugar is a problem for you. See " What are the possible side effects of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets? "
- Check your blood sugar as your healthcare provider tells you to.
- Stay on your prescribed diet and exercise program while taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets.
- If you miss a dose of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, take your next dose as prescribed unless your healthcare provider tells you differently. Do not take an extra dose the next day.
- If you take too much metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, call your healthcare provider, local Poison Control Center, or go to the nearest hospital emergency room right away.
What should I avoid while taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
Do not drink a lot of alcoholic drinks while taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets. This means you should not binge drink for short periods, and you should not drink a lot of alcohol on a regular basis. Alcohol can increase the chance of getting lactic acidosis.
What are the side effects of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
- Lactic acidosis. Metformin, the active ingredient in metformin hydrochloride tablets and metformin hydrochloride extended-release tablets, can cause a rare but serious condition called lactic acidosis (a buildup of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Call your doctor right away if you have any of the following symptoms, which could be signs of lactic acidosis:
- you feel cold in your hands or feet
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
- you feel very weak or tired
- you have trouble breathing
- you feel sleepy or drowsy
- you have stomach pains, nausea or vomiting
Most people who have had lactic acidosis with metformin have other things that, combined with the metformin, led to the lactic acidosis. Tell your doctor if you have any of the following, because you have a higher chance for getting lactic acidosis with metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if you:
- have severe kidney problems, or your kidneys are affected by certain x-ray tests that use injectable dye
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids
- have surgery
- have a heart attack, severe infection, or stroke
Common side effects of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets include diarrhea, nausea, and upset stomach. These side effects generally go away after you take the medicine for a while. Taking your medicine with meals can help reduce these side effects. Tell your doctor if the side effects bother you a lot, last for more than a few weeks, come back after they've gone away, or start later in therapy. You may need a lower dose or need to stop taking the medicine for a short period or for good.
About 3 out of every 100 people who take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets have an unpleasant metallic taste when they start taking the medicine. It lasts for a short time.
Metformin hydrochloride tablets or metformin hydrochloride extended-release tablets rarely cause hypoglycemia (low blood sugar) by themselves. However, hypoglycemia can happen if you do not eat enough, if you drink alcohol, or if you take other medicines to lower blood sugar.
How should I store metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Store metformin hydrochloride tablets and metformin hydrochloride extended-release tablets at 20° to 25°C (68° to 77°F).
Keep metformin hydrochloride tablets and metformin hydrochloride extended-release tablets and all medicines out of the reach of children.
General information about the use of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets
If you have questions or problems, talk with your doctor or other healthcare provider. You can ask your doctor or pharmacist for the information about metformin hydrochloride tablets and metformin hydrochloride extended-release tablets that is written for healthcare professionals. Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use metformin hydrochloride tablets or metformin hydrochloride extended-release tablets for a condition for which it was not prescribed. Do not share your medicine with other people.
What are the ingredients of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Active ingredients of metformin hydrochloride tablets: metformin hydrochloride, USP.
Inactive ingredients in each tablet of metformin hydrochloride tablets: colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone and sodium starch glycolate.
Active ingredients of metformin hydrochloride extended-release tablets: metformin hydrochloride, USP.
Inactive ingredients in each tablet of metformin hydrochloride extended-release tablets 500 mg and 750 mg: glyceryl behenate, hypromellose, microcrystalline cellulose and povidone.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level. High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Talk to your healthcare provider about how to prevent, recognize, and take care of low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and problems you have because of your diabetes.
Other brands listed are the trademarks of their respective owners.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
| METFORMIN HYDROCHLORIDE
metformin hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PD-Rx Pharmaceuticals, Inc. | 156893695 | repack(43063-507) | |