IVIZIA DRY EYE- povidone solution/ drops
iVIZIA Dry Eye by
Drug Labeling and Warnings
iVIZIA Dry Eye by is a Otc medication manufactured, distributed, or labeled by Thea Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
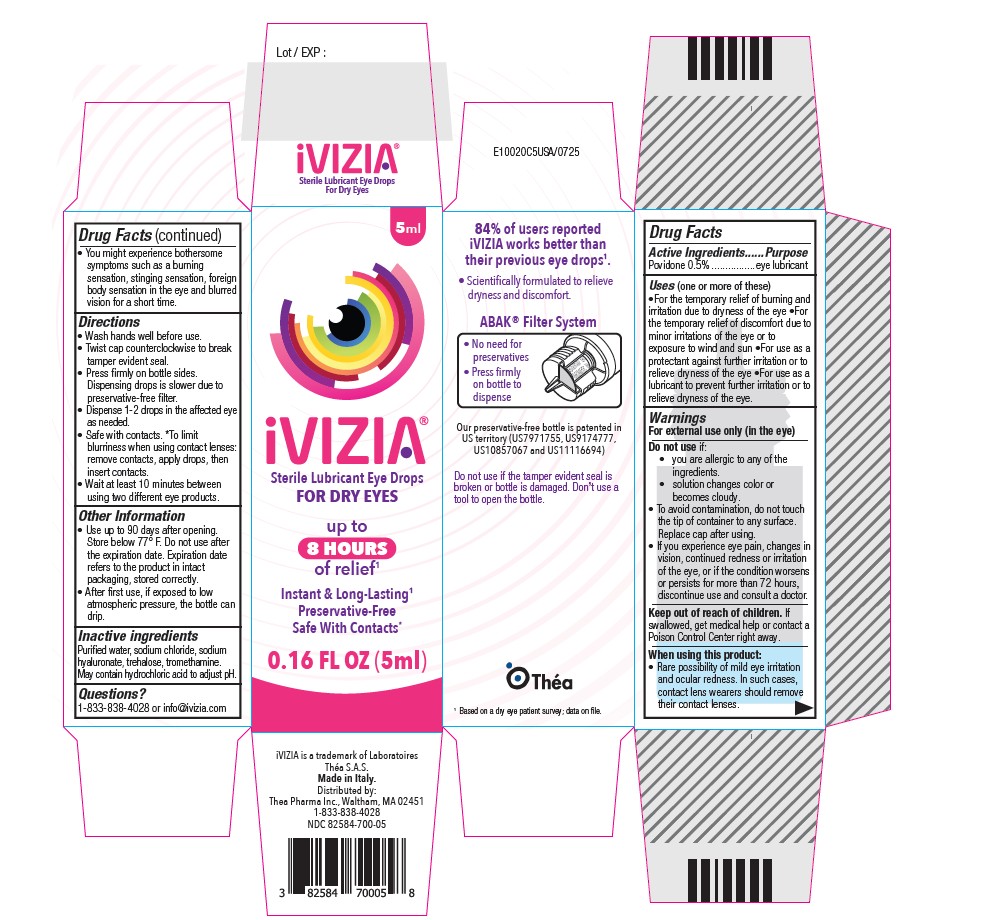
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
(one or more of these)
For the temporary relief of burning and irritation due to dryness of the eye
For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind and sun
For use as a protectant against further irritation or to relieve dryness of the eye
For use as a lubricant to prevent further irritation or to relieve dryness of the eye. -
Warnings
For external use only(in the eye)
Do not use if:
you are allergic to any of the ingredients.
solution changes color or becomes cloudy.
To avoid contamination, do not touch the tip of container to any surface.
Replace cap after using.
If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor.Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
When using this product
Rare possibility of mild eye irritation and ocular redness. In such cases, contact lens wearers should remove their contact lenses.
You might experience bothersome symptoms such as a burning sensation, stinging sensation, foreign body sensation in the eye and blurred vision for a short time. -
Directions:
Wash hands well before use.
Twist cap counterclockwise to break tamper evident seal.
Press firmly on bottle sides. Dispensing drops is slower due to preservative-free filter.
Dispense 1-2 drops in the affected eye as needed.
Safe with contacts. *To limit blurriness when using contact lenses: remove contacts, apply drops, then insert contacts. - Other Information
- Inactive Ingredients
- Questions?
- Principle Display Panel
-
INGREDIENTS AND APPEARANCE
IVIZIA DRY EYE
povidone solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82584-700 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE (UNII: FZ989GH94E) (POVIDONE - UNII:FZ989GH94E) POVIDONE 25 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) TREHALOSE (UNII: B8WCK70T7I) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82584-700-05 1 in 1 CARTON 12/01/2024 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 82584-700-11 1 in 1 CARTON 12/01/2024 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 82584-700-13 1 in 1 CARTON 12/01/2024 3 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/01/2024 Labeler - Thea Pharma Inc. (117787029)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.