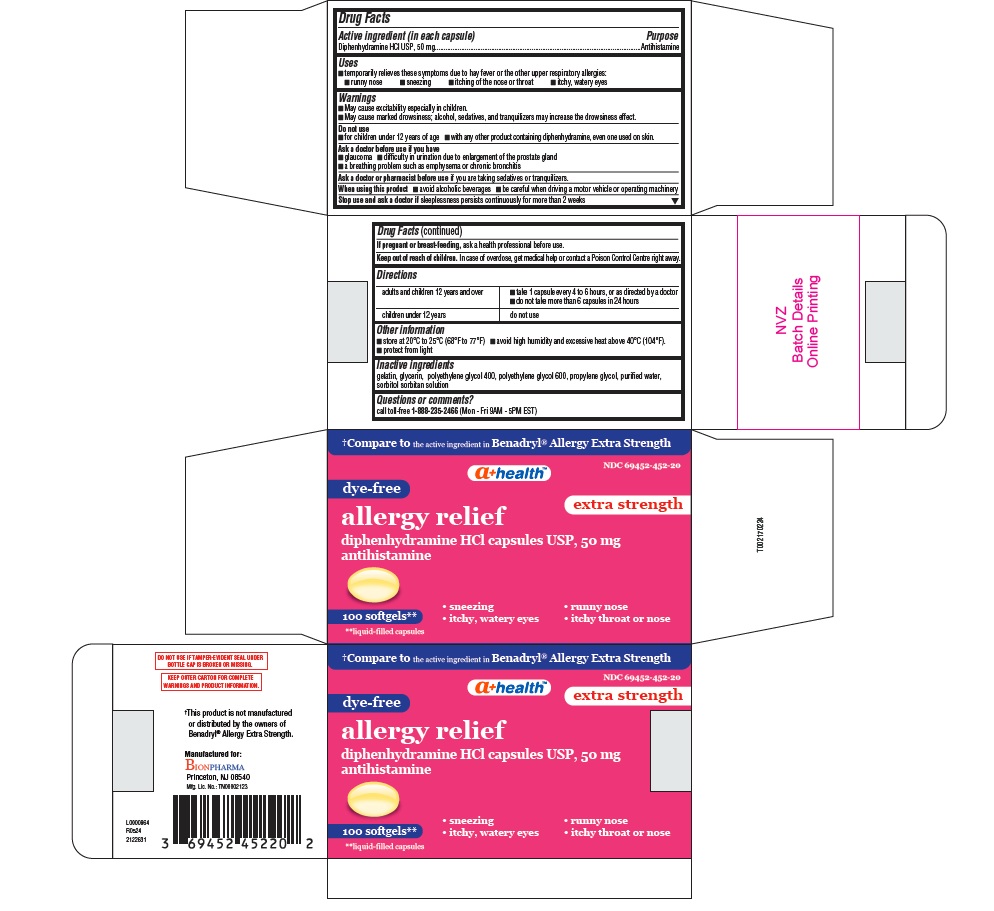
ALLERGY RELIEF- diphenhydramine hcl capsule, liquid filled
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by BIONPHARMA INC., Bionpharma Inc., SOFTGEL HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each capsule)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast- feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
DO NOT USE IF TAMPER-EVIDENT SEAL UNDER BOTTLE CAP IMPRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
†This product is not manufactured or distributed by the owners of Benadryl® Allergy Extra Strength.
Manufactured for:
Bionpharma
Princeton, NJ 08540
Mfg. Lic. No.: TN00002123
L0000864
R0524
2122631 -
100's count
†Compare to the active ingredient in Benadryl® Allergy Extra Strength
NDC: 69452-452-20
a+ health TM
dye-free
extra strength
allergy relief
diphenhydramine HCl capsules USP, 50 mg
antihistamine
sneezing runny nose
itchy, watery eyes itchy throat or nose100 softgels **
**liquid-filled capsules
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69452-452 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) SORBITAN (UNII: 6O92ICV9RU) GELATIN (UNII: 2G86QN327L) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white (Clear Transparent) Score no score Shape OVAL Size 13mm Flavor Imprint Code DF50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-452-20 1 in 1 CARTON 10/01/2024 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 69452-452-83 300 in 1 BOTTLE; Type 0: Not a Combination Product 03/02/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/01/2024 Labeler - BIONPHARMA INC. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PRIVATE LIMITED 676666501 manufacture(69452-452)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.