FORTE MAGNETIC S3 OIL CONTROL ANTI-DANDFUFF- piroctone olamine lotion/shampoo
FORTE Magnetic S3 Oil Control Anti-Dandfuff by
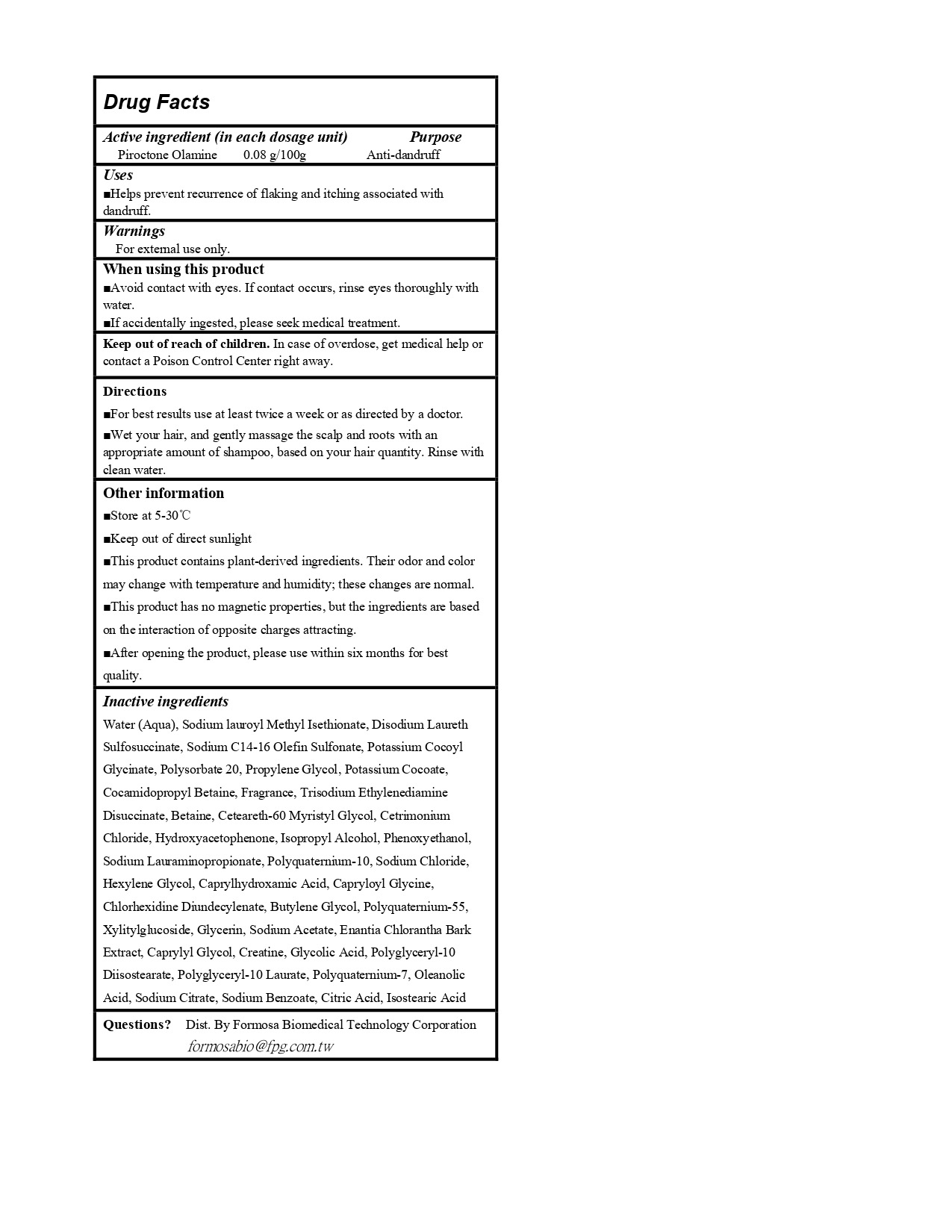
Drug Labeling and Warnings
FORTE Magnetic S3 Oil Control Anti-Dandfuff by is a Otc medication manufactured, distributed, or labeled by FORMOSA BIOMEDICAL TECHNOLOGY CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
OTHER SAFETY INFORMATION
Other information:
Store at 5-30C;
Keep out of direct sunlight;
This product contains plant-derived ingredients.Their odor and color may change with temperature and humidity; these changes are nomal;
This product has no magnetic properties, but the ingredients are based on the interaction of opposite charges attracting. -
INACTIVE INGREDIENT
Inactive ingredients:
Water (Aqua), Sodium lauroyl Methyl Isethionate, Disodium Laureth Sulfosuccinate, Sodium C14-16 Olefin Sulfonate, Potassium Cocoyl Glycinate, Polysorbate 20, Propylene Glycol, Potassium Cocoate, Cocamidopropyl Betaine, Fragrance, Trisodium Ethylenediamine Disuccinate, Betaine, Ceteareth-60 Myristyl Glycol, Cetrimonium Chloride, Hydroxyacetophenone, Isopropyl Alcohol, Phenoxyethanol, Sodium Lauraminopropionate, Polyquaternium-10, Sodium Chloride, Hexylene Glycol, Caprylhydroxamic Acid, Capryloyl Glycine, Butylene Glycol, Polyquaternium-55, Xylitylglucoside, Glycerin, Sodium Acetate, Enantia Chlorantha Bark Extract, Caprylyl Glycol, Creatine, Glycolic Acid, Polyglyceryl-10 Laurate, Polyquaternium-7, Oleanolic Acid, Sodium Citrate, Sodium Benzoate, Citric Acid, Isostearic Acid
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FORTE MAGNETIC S3 OIL CONTROL ANTI-DANDFUFF
piroctone olamine lotion/shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 84655-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PIROCTONE OLAMINE (UNII: A4V5C6R9FB) (PIROCTONE - UNII:R49EFA73Q7) PIROCTONE OLAMINE 0.4 g in 500 g Inactive Ingredients Ingredient Name Strength DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) ANNICKIA CHLORANTHA BARK (UNII: H70115MP4A) POTASSIUM COCOYL GLYCINATE (UNII: WZ70FUF22U) POTASSIUM COCOATE (UNII: F8U72V8ZXP) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 20 (UNII: 7T1F30V5YH) CREATINE (UNII: MU72812GK0) HEXYLENE GLYCOL (UNII: KEH0A3F75J) POLYQUATERNIUM-10 (400 CPS AT 2%) (UNII: HB1401PQFS) SODIUM LAUROYL METHYL ISETHIONATE (UNII: II6VCD3S6R) SODIUM CITRATE (UNII: 1Q73Q2JULR) POLYQUATERNIUM-55 (UNII: NG3KW6CD2J) OLEANOLIC ACID (UNII: 6SMK8R7TGJ) SODIUM ACETATE (UNII: 4550K0SC9B) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) BETAINE (UNII: 3SCV180C9W) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600 KD) (UNII: 0L414VCS5Y) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) ISOPROPYL ALCOHOL (UNII: ND2M416302) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GLYCOLIC ACID (UNII: 0WT12SX38S) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) FRAGRANCE 13576 (UNII: 5EM498GW35) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) GLYCERIN (UNII: PDC6A3C0OX) CETEARETH-60 MYRISTYL GLYCOL (UNII: 4O832ZOY6W) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SODIUM LAURAMINOPROPIONATE (UNII: X5NJA9HXPU) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84655-101-02 1 in 1 BOX 08/13/2024 1 NDC: 84655-101-01 500 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/13/2024 Labeler - FORMOSA BIOMEDICAL TECHNOLOGY CORPORATION (658521104) Establishment Name Address ID/FEI Business Operations FORMOSA BIOMEDICAL TECHNOLOGY CORPORATION 658521104 manufacture(84655-101)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.