84641-002 (Hand Sanitizer )
Hand Sanitizer by
Drug Labeling and Warnings
Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by NANTONG QINJI HEALTH TECHNOLOGY CO., LTD, NANTONG QINJI HEALTH TECHNOLOGY CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
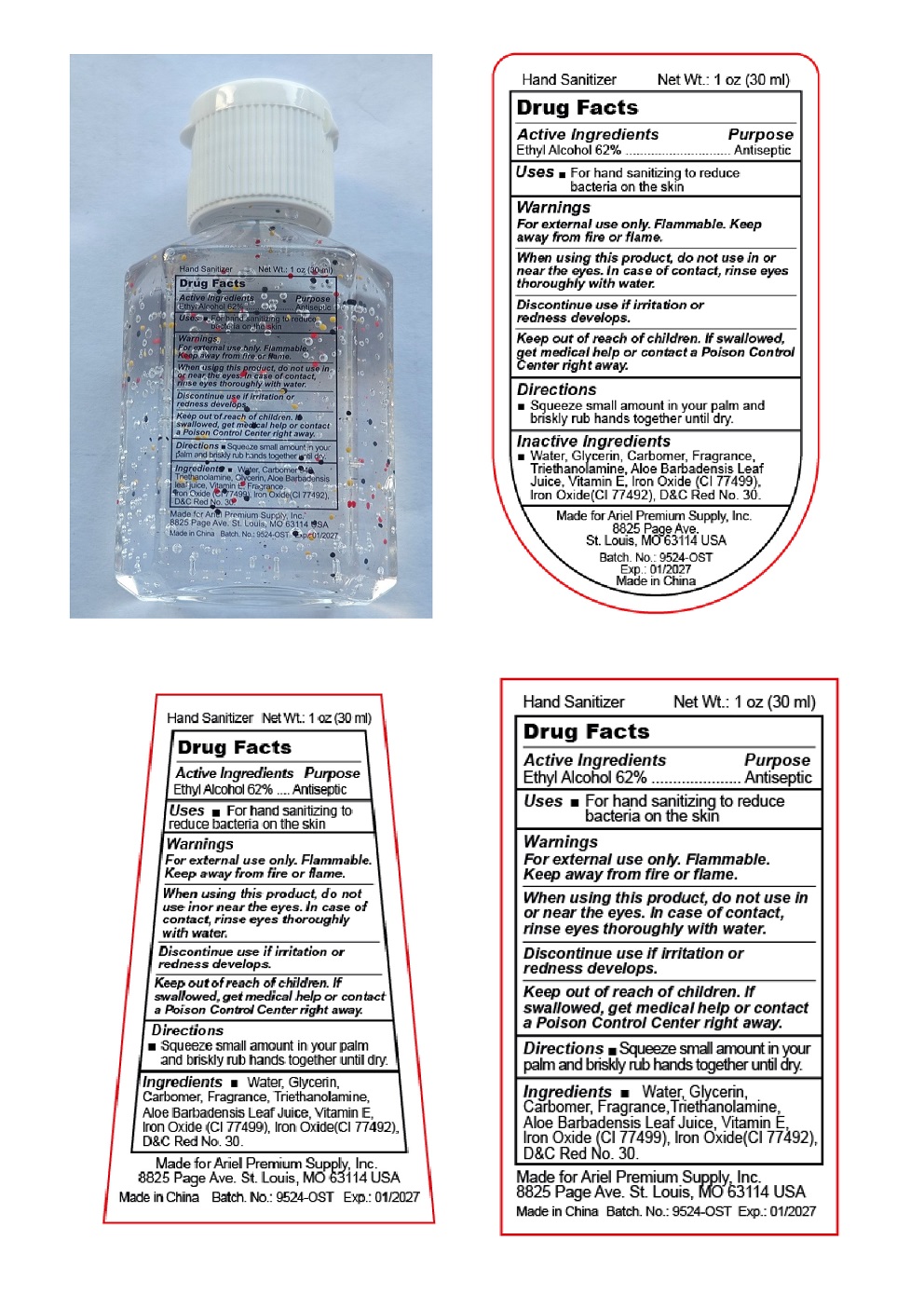
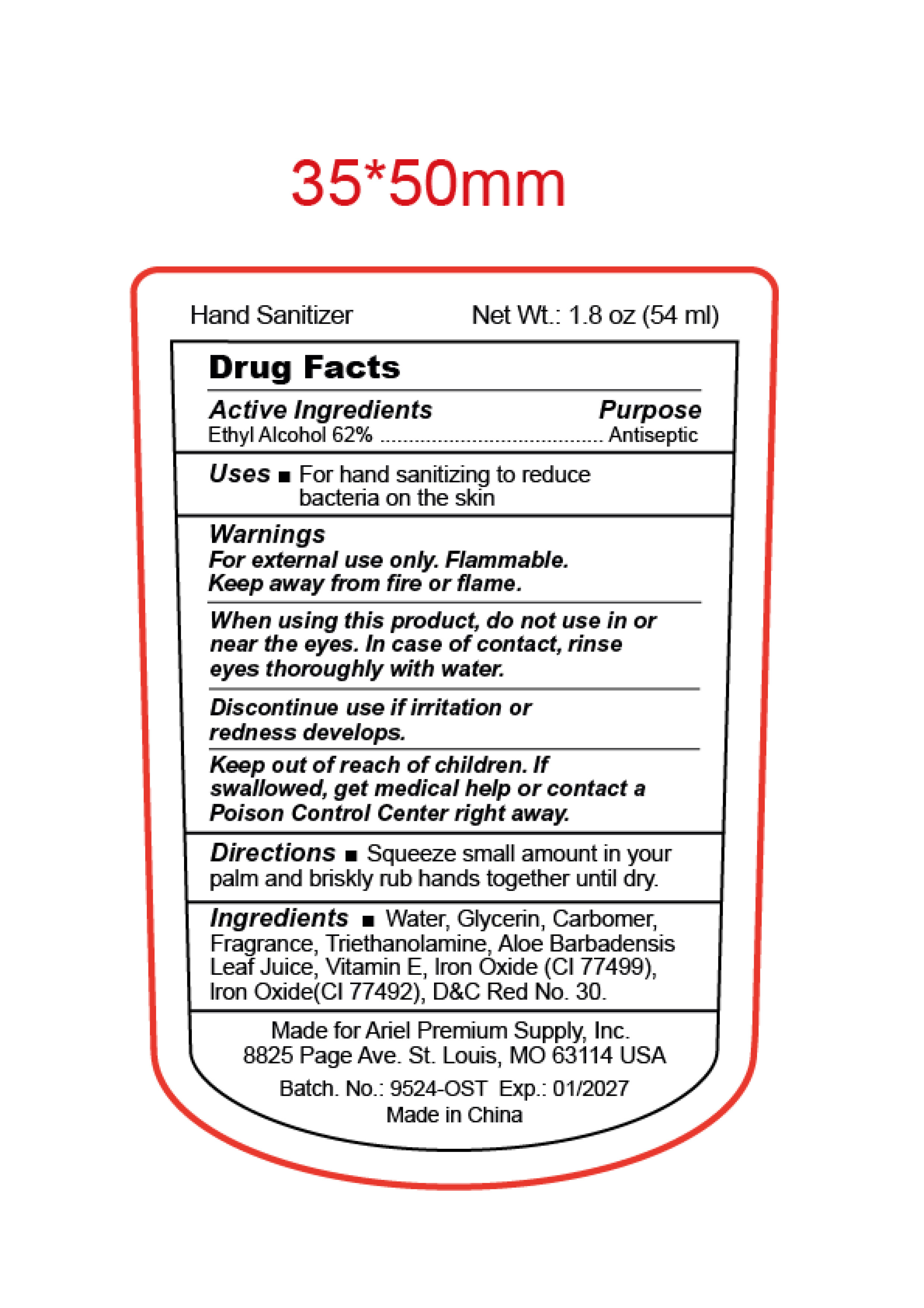
HAND SANITIZER- alcohol gel
NANTONG QINJI HEALTH TECHNOLOGY CO., LTD
----------
84641-002 (Hand Sanitizer)
Warinings
For external use only.
Flammable. Keep away from fire and flame.
| HAND SANITIZER
alcohol gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - NANTONG QINJI HEALTH TECHNOLOGY CO., LTD (450420747) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NANTONG QINJI HEALTH TECHNOLOGY CO., LTD. | 450420747 | manufacture(84641-002) | |
Revised: 8/2024
Document Id: 2013f1e9-94f3-348f-e063-6394a90aa948
Set id: 1f9ea65c-bd8e-e25b-e063-6294a90a0d20
Version: 3
Effective Time: 20240819
Trademark Results [Hand Sanitizer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HAND SANITIZER 88958909 not registered Live/Pending |
MAISON BLANCHE, LLC 2020-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.