RIMMEL BB CREAM 9-IN-1 SKIN PERFECTING SUPER MAKEUP LIGHT MEDIUM- avobenzone, octinoxate, octisalate, octocrylene lotion
Rimmel BB Cream 9-IN-1 Skin Perfecting Super Makeup by
Drug Labeling and Warnings
Rimmel BB Cream 9-IN-1 Skin Perfecting Super Makeup by is a Otc medication manufactured, distributed, or labeled by Rimmel Inc., Coty Lancaster S.A.M.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
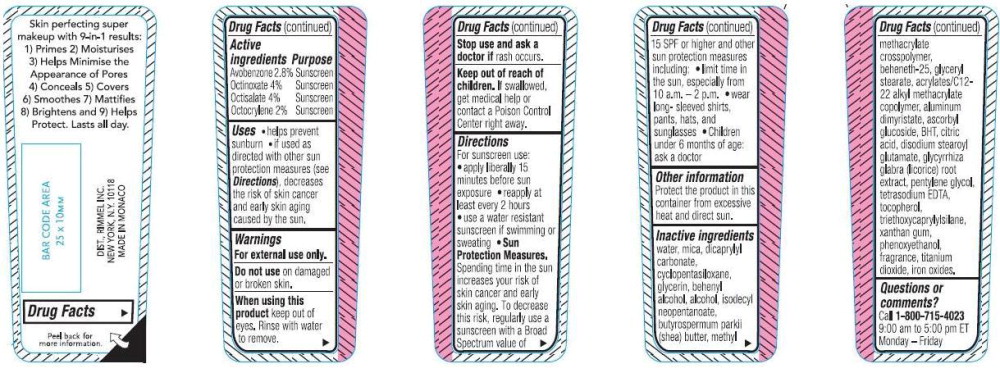
- Drug Facts
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum value of 15 SPF or higher and other sun protection measures including:
- Limit tim in the sun, especially from 10 a.m-2p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: ask a doctor.
-
INACTIVE INGREDIENT
water, dicaprylyl carbonate, mica, cyclopentasiloxane, glycerin, behenyl alcohol, alcohol, isodecyl neopentanoate, butyrospermum parkii (shea) butter, methyl methacrylate crosspolymer, beheneth-25, glyceryl stearate, acrylates/C12-22 alkyl methacrylate copolymer, aluminum dimyristate, ascorbyl glucoside, BHT, citric acid, disodium stearoyl glutamate, glycyrrhiza glabra (licorice) root extract, pentylene glycol, tetrasodium EDTA, tocopherol, triethoxycaprylylsilane, xanthan gum, phenoxethanol, fragrance, titanium dioxide, iron oxides.
- BB Cream Beauty Balm SPF 25 Sunscreen Light/Medium
- Drug Facts Box
-
INGREDIENTS AND APPEARANCE
RIMMEL BB CREAM 9-IN-1 SKIN PERFECTING SUPER MAKEUP LIGHT MEDIUM
avobenzone, octinoxate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76485-1048 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.9 g in 30 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.3 g in 30 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 1.3 g in 30 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.6 in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) MICA (UNII: V8A1AW0880) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) DOCOSANOL (UNII: 9G1OE216XY) ALCOHOL (UNII: 3K9958V90M) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) SHEA BUTTER (UNII: K49155WL9Y) METHYL METHACRYLATE (UNII: 196OC77688) BEHENETH-25 (UNII: 0G17KJ5M7P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) LICORICE (UNII: 61ZBX54883) PENTYLENE GLYCOL (UNII: 50C1307PZG) EDETATE SODIUM (UNII: MP1J8420LU) TOCOPHEROL (UNII: R0ZB2556P8) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76485-1048-1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2014 Labeler - Rimmel Inc. (401011325) Establishment Name Address ID/FEI Business Operations Coty Lancaster S.A.M. 401011325 manufacture(76485-1048)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.