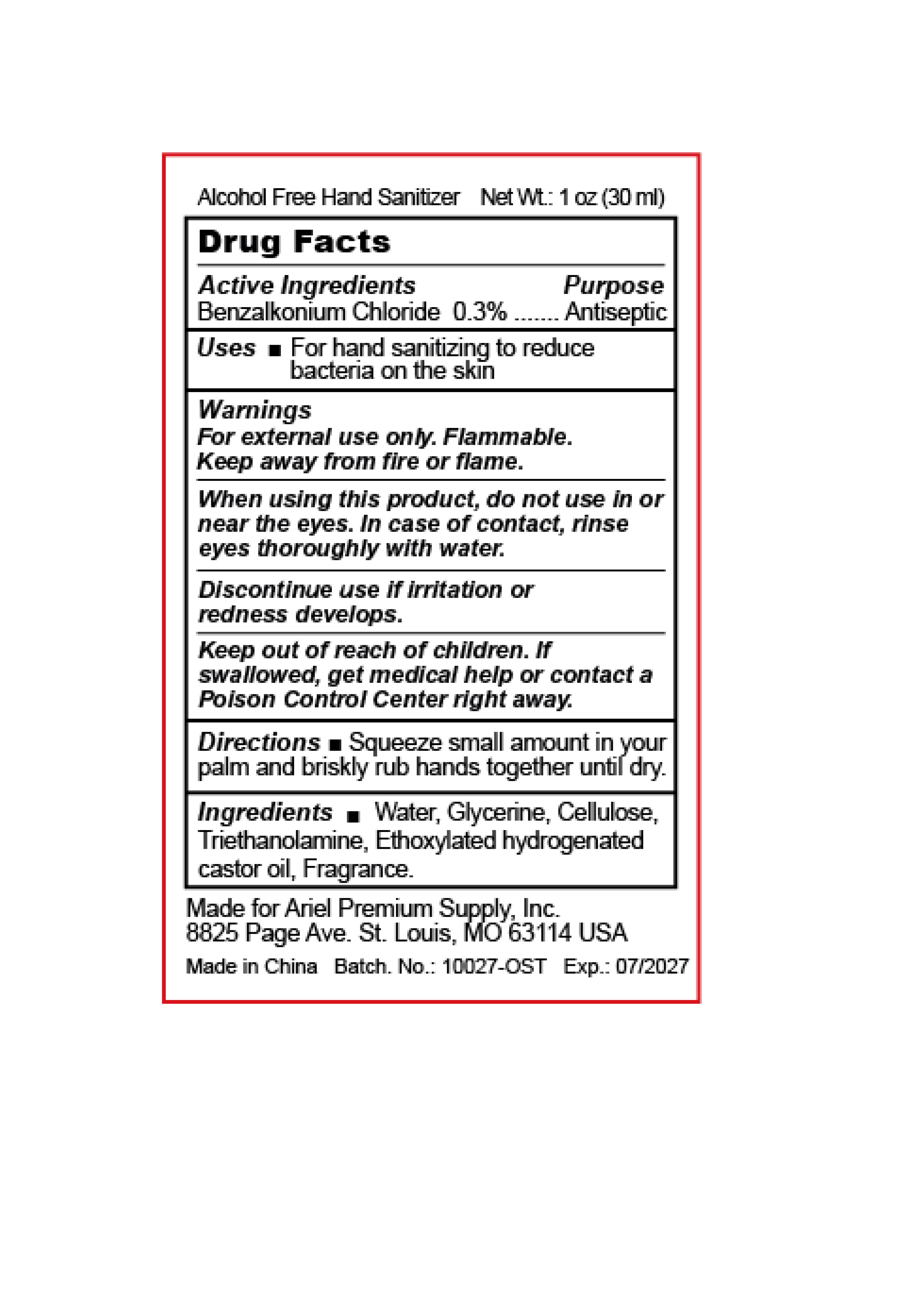
84641-004 (Alcohol Free Hand Sanitizer )
Alcohol Free Hand Sanitizer by
Drug Labeling and Warnings
Alcohol Free Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by NANTONG QINJI HEALTH TECHNOLOGY CO., LTD, NANTONG QINJI HEALTH TECHNOLOGY CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALCOHOL FREE HAND SANITIZER- benzalkonium chloride gel
NANTONG QINJI HEALTH TECHNOLOGY CO., LTD
----------
84641-004 (Alcohol Free Hand Sanitizer)
Warinings
For external use only.
Flammable. Keep away from fire and flame.
| ALCOHOL FREE HAND SANITIZER
benzalkonium chloride gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - NANTONG QINJI HEALTH TECHNOLOGY CO., LTD (450420747) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NANTONG QINJI HEALTH TECHNOLOGY CO., LTD. | 450420747 | manufacture(84641-004) | |
Revised: 8/2024
Document Id: 20140907-a457-4d46-e063-6294a90af018
Set id: 1fa05d6c-0ad7-9986-e063-6294a90aeb26
Version: 3
Effective Time: 20240819
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.