Eyewash by MWI / Akorn Operating Company LLC / Niagara Pharmaceuticals, Inc. EYEWASH- water solution
Eyewash by
Drug Labeling and Warnings
Eyewash by is a Otc medication manufactured, distributed, or labeled by MWI, Akorn Operating Company LLC, Niagara Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
- Directions
- Other information
- Inactive ingredients
- Questions ?
-
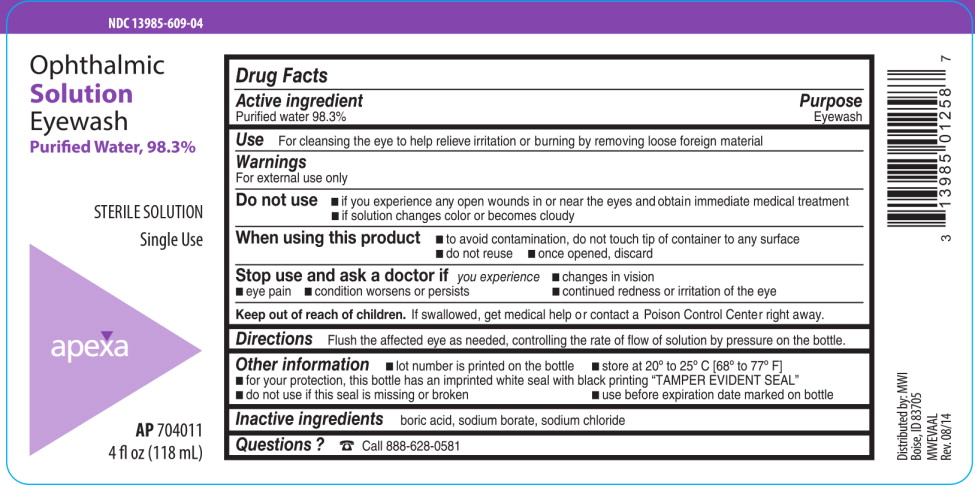
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 13985-609-04
Ophthalmic
Solution
Eyewash
Purified Water, 98.3%
STERILE SOLUTION
Single Use
APEXA logo
AP 704011
4 fl.oz. (118 mL)
-
INGREDIENTS AND APPEARANCE
EYEWASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13985-609 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 929 g in 946 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13985-609-04 1 in 1 BOTTLE 03/25/2015 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 02/23/2015 Labeler - MWI (019926120) Registrant - Niagara Pharmaceuticals, Inc. (205477792) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, Inc. 205477792 manufacture(13985-609)
Trademark Results [Eyewash]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EYEWASH 75397229 2262356 Dead/Cancelled |
Sync, Inc., The 1997-11-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.