BETAMETHASONE VALERATE aerosol, foam
Betamethasone Valerate by
Drug Labeling and Warnings
Betamethasone Valerate by is a Prescription medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Betamethasone foam contains betamethasone valerate, a synthetic corticosteroid, for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory agents.
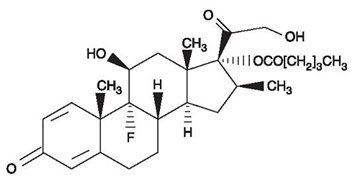
Betamethasone valerate is 9-fluoro11ß,17, 21-trihydroxy-16ß-methylpregna-1, 4-diene-3, 20-dione 17-valerate, with the molecular formula C27H37FO6, a molecular weight of 476.58. The following is the chemical structure:
Betamethasone valerate, USP is a white to practically white, odorless crystalline powder, and is practically insoluble in water, freely soluble in acetone and in chloroform, soluble in alcohol, and slightly soluble in benzene and in ether.
Betamethasone valerate foam, 0.12%, contains 1.2 mg betamethasone valerate, per gram in a thermolabile hydroethanolic foam vehicle consisting of cetyl alcohol, citric acid, ethanol (60.4%), polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol pressurized with a hydrocarbon (propane/butane) propellant.
-
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, betamethasone valerate foam has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption.
The use of pharmacodynamic endpoints for assessing the systemic exposure of topical corticosteroids is necessary due to the fact that circulating levels are well below the level of detection. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. They are metabolized, primarily in the liver, and are then excreted by the kidneys. In addition, some corticosteroids and their metabolites are also excreted in the bile.
-
CLINICAL STUDIES
The safety and efficacy of betamethasone valerate foam has been demonstrated in a four-week trial. An adequate and well-controlled clinical trial was conducted in 190 patients with moderate to severe scalp psoriasis. Patients were treated twice daily for four weeks with betamethasone valerate foam, placebo foam, a commercially available betamethasone valerate lotion 0.12% (formerly expressed as 0.1% betamethasone), or placebo lotion. At four weeks of treatment, study results of 159 patients demonstrated that the efficacy of betamethasone valerate foam in treating scalp psoriasis is superior to that of placebo foam, and is comparable to that of a currently marketed BMV lotion (see Table below).
Subjects with Target Lesion Parameter Clear at Endpoint
Betamethasone Valerate Foam
n (%)
BMV lotion
n (%)
Placebo foam
n (%)
Scaling
30 (47%)
22 (35%)
2 (6%)
Erythema
26 (41%)
16 (25%)
2 (6%)
Plaque Thickness
42 (66%)
25 (40%)
5 (16%)
Investigator's Global: Subjects Completely Clear or Almost Clear at Endpoint
43 (67%)
29 (46%)
6 (19%)
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Systemic absorption of topical corticosteroids has caused reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings.
Therefore, patients applying a topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios. (See PRECAUTIONS-Pediatric Use.)
If irritation develops, betamethasone valerate foam should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noting a clinical exacerbation, as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, use of betamethasone valerate foam should be discontinued until the infection has been adequately controlled.
Information for Patients
Patients using topical corticosteroids should receive the following information and instructions:
- 1. This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- 2. This medication should not be used for any disorder other than that for which it was prescribed.
- 3. The treated scalp area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- 4. Patients should report to their physician any signs of local adverse reactions.
- 5. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, contact the physician.
Laboratory Tests
The following tests may be helpful in evaluating patients for HPA axis suppression:
ACTH stimulation test
A.M. plasma cortisol test
Urinary free cortisol testCarcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of betamethasone valerate.
Betamethasone was genotoxic in the in vitro human peripheral blood lymphocyte chromosome aberration assay with metabolic activation and in the in vivo mouse bone marrow micronucleus assay.
Pregnancy Category C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women. Therefore, betamethasone valerate foam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, caution should be exercised when betamethasone valerate foam is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
-
ADVERSE REACTIONS
The most frequent adverse event was burning/itching/stinging at the application site; the incidence and severity of this event were as follows:
Incidence and severity of burning/itching/stinging
Maximum severity
Product
Total incidence
Mild
Moderate
Severe
Betamethasone valerate foam n = 63
34 (54%)
28 (44%)
5 (8%)
1 (2%)
Betamethasone valerate lotion n = 63
33 (52%)
26 (41%)
6 (10%)
1 (2%)
Placebo Foam n = 32
24 (75%)
13 (41%)
7 (22%)
4 (12%)
Placebo Lotion n = 30
20 (67%)
12 (40%)
5 (17%)
3 (10%)
Other adverse events which were considered to be possibly, probably, or definitely related to betamethasone valerate foam occurred in 1 patient each; these were paresthesia, pruritus, acne, alopecia, and conjunctivitis.
The following additional local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximately decreasing order of occurrence: irritation; dryness; folliculitis; acneiform eruptions; hypopigmentation; perioral dermatitis; allergic contact dermatitis; secondary infection; skin atrophy; striae; and miliaria.
Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria in some patients.
-
OVERDOSAGE
Topically applied betamethasone valerate foam can be absorbed in sufficient amounts to produce systemic effects. (See PRECAUTIONS.)
-
DOSAGE AND ADMINISTRATION
Note: For proper dispensing of foam, can must be inverted.
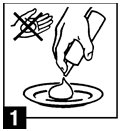
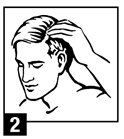
For application to the scalp invert can and dispense a small amount of betamethasone valerate foam onto a saucer or other cool surface. Do not dispense directly onto hands as foam will begin to melt immediately upon contact with warm skin. Pick up small amounts of foam with fingers and gently massage into affected area until foam disappears. Repeat until entire affected scalp area is treated. Apply twice daily, once in the morning and once at night.
As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Betamethasone valerate foam should not be used with occlusive dressings unless directed by a physician.
-
HOW SUPPLIED
Betamethasone Valerate Foam, 0.12% contains 1.2 mg of betamethasone valerate, USP per gram. It is available as follows:
NDC: 0378-8180-50
50 g aluminum canNDC: 0378-8180-99
100 g aluminum canStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
WARNING
FLAMMABLE. AVOID FIRE, FLAME OR SMOKING DURING AND IMMEDIATELY FOLLOWING APPLICATION. Keep out of reach of children. Contents under pressure. Do not puncture or incinerate container. Do not expose to heat or store at temperatures above 120°F (49°C).
Questions: Call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX). Side effects should be reported to this number.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215140880-0317
Revised: 3/2017
DPT:BETA:R1p -
PATIENT INFORMATION
Betamethasone Valerate Foam
0.12%
(bay″ ta meth′ a sone val′ er ate)About Betamethasone Valerate Foam
Your doctor has prescribed betamethasone valerate foam, 0.12%, for the relief of corticosteroid-responsive skin conditions of the scalp. Betamethasone valerate foam works because its active ingredient is betamethasone valerate, 0.12%. Betamethasone belongs to a group of medicines known as topical corticosteroids. These agents are used to reduce the inflammation, redness, swelling, itching, and tenderness associated with dermatologic conditions.
Other ingredients in betamethasone valerate foam include cetyl alcohol, citric acid, ethanol, polysorbate 60, potassium citrate, propylene glycol, purified water, and stearyl alcohol. The foam is dispensed from an aluminum can that is pressurized by a hydrocarbon propellant (propane and butane).
If you answer YES to one or more of the following questions, tell your doctor (or pharmacist) before using this medicine, so you can get advice about what to do.
- Are you allergic to any of the ingredients contained in betamethasone valerate foam?
- Are you pregnant? Planning on becoming pregnant while using betamethasone valerate foam? Or are you breastfeeding?
- Do you think you have an infection on your scalp?
- Do not use this medication for any condition other than the one for which it was prescribed.
- Betamethasone valerate foam is for external use only.
- Keep the foam away from your eyes, as it will sting. If the foam gets into your eyes, rinse well with cold water. If the stinging continues, contact your doctor immediately.
WHAT YOU SHOULD KNOW ABOUT BETAMETHASONE VALERATE FOAM:
What to do if you miss an application
If you forget to apply betamethasone valerate foam at the scheduled time, use it as soon as you remember, and then go back to your regular schedule. If you remember at or about the time of your next daily application, apply that dose and continue with your normal application schedule. If you miss several doses, tell your doctor at your next appointment.
About side effects
As with all medications, there may be some side effects. The most frequent side effects associated with the use of betamethasone valerate foam include mild burning, stinging, or itching at the site of application. These side effects typically disappear shortly after application.
Let your doctor know if you notice any of the following:
- Any unusual effects that you do not understand.
- Affected areas that do not seem to be healing after several weeks of using the foam.
Important safety notes
- The treated areas should not be bandaged or covered unless directed by your doctor.
- Keep this and all medicines out of the reach of children.
- Store the can at controlled room temperature 20° to 25°C (68° to 77°F) and protect it from direct sunlight, as this is a pressurized container.
- Keep away from and do not spray near fire, open flame, or direct heat--this product is flammable. Do not smoke while using or holding the can. Keep the can away from all sources of ignition. Do not pierce or burn the can, and never throw the can in a fire, even if empty.
- When you have finished your treatment, dispose of the can safely. A completely empty can is recyclable.
- Do not use the foam after the expiration date shown on the bottom of the can.
- Do not give betamethasone valerate foam to anyone else. Your doctor has prescribed this medicine for your use only.
For additional information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215Revised: 3/2017
PL:DPT:BETA:R1p -
PRINCIPAL DISPLAY PANEL – 0.12%
NDC: 0378-8180-50
Betamethasone
Valerate
Foam
0.12%Rx only 50 g
FOR DERMATOLOGIC USE ONLY.
NOT FOR OPHTHALMIC USE.Invert can
and then
press firmly
to dispenseDescription: Betamethasone
valerate foam, 0.12%, contains
1.2 mg betamethasone valerate,
USP, per gram in a thermolabile
hydroethanolic foam vehicle
consisting of cetyl alcohol, citric
acid, ethanol (60.4%), polysorbate
60, potassium citrate, propylene
glycol, purified water, and stearyl
alcohol pressurized with a
hydrocarbon (propane/butane)
propellant.Dosage: See package insert for full
prescribing information. For
application to the scalp, invert can
and dispense a small amount of
betamethasone valerate foam onto
a saucer or other cool surface. (Do
not dispense directly onto hands, as
foam will begin to melt immediately
upon contact with warm skin.) Pick
up small amounts of foam with
fingers and gently massage into
affected area until foam
disappears. Repeat until entire
affected scalp area is treated. Apply
twice daily, once in the morning
and once at night.Warning: FLAMMABLE. AVOID
FIRE, FLAME, OR SMOKING
DURING AND IMMEDIATELY
FOLLOWING APPLICATION.Keep away from eyes or other
mucous membranes. Keep out of
reach of children.Contents under pressure. Do not
puncture or incinerate container.
Do not expose to heat or store at
temperatures above 120°F (49°C).Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]DPT:8180:50:1C:R1
117459-0317
CFC FREE
Questions:
Call Mylan Pharmaceuticals
Inc. at 1-877-446-3679
(1-877-4-INFO-RX)Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215 U.S.A.Mylan.com
-
INGREDIENTS AND APPEARANCE
BETAMETHASONE VALERATE
betamethasone valerate aerosol, foamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0378-8180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BETAMETHASONE VALERATE (UNII: 9IFA5XM7R2) (BETAMETHASONE - UNII:9842X06Q6M) BETAMETHASONE VALERATE 1.2 mg in 1 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-8180-50 50 g in 1 CAN; Type 0: Not a Combination Product 04/25/2017 2 NDC: 0378-8180-99 100 g in 1 CAN; Type 0: Not a Combination Product 04/25/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA020934 04/25/2017 Labeler - Mylan Pharmaceuticals Inc. (059295980)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.