0.025% Capsaicin Patch (2 Patches/Pouch) by Koolcare Technology Co., Ltd
0.025% Capsaicin Patch (2 Patches/Pouch) by
Drug Labeling and Warnings
0.025% Capsaicin Patch (2 Patches/Pouch) by is a Otc medication manufactured, distributed, or labeled by Koolcare Technology Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
3% MENTHOL PLUS 0.083% CAPSAICIN PAIN RELIEF PATCH (2 PATCHES/POUCH) - pain relief patch, pain relief strip patch
5% MENTHOL PAIN RELIEF PATCH (1 LARGE PATCH/POUCH)- pain relief patch patch
5% MENTHOL PAIN RELIEF PATCH (2 PATCHES/POUCH) - pain relief patch patch
0.025% CAPSAICIN PATCH (2 PATCHES/POUCH) 82632- capsaicin pain relief patch, capsaicin pain relief strip patch
Koolcare Technology Co., Ltd
----------
0.025% Capsaicin Patch (2 Patches/Pouch) NDC: 84205-005-00

Uses
Topical Analgesic
For temporary relief of minor aches and pains of muscles & joints associated with:
- Simple Backache
- Arthritis
- Strains
- Bruises
- Sprains
Warnings
WARNINGS: EXTERNAL USE ONLY
DO NOT USE
- on wounds or damaged skin
- with a heating pad
- with or at the same times as other external analgesic
- if you are allergic to any ingredients of this product
When using this product
- do not use otherwise than as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
- discountinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
STOP USE and ask a doctor if
- rash, itching or excessive skin irritation develops
- conditions worsen
- symptoms persist for more than 7 days
- symptoms clear up and occur again with few days
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Propylene Glycol, Polysorbate 80, Carboxymethylcellulose Sodium, Carbomer, Purified Water, POVIDONE K90, Tartaric Acid, DMDM Hydantoin
0.025% Capsaicin Patch (24 Pouches/Box) NDC: 84205-005-01

Uses
Topical Analgesic
For temporary relief of minor aches and pains of muscles & joints associated with:
- Simple Backache
- Arthritis
- Strains
- Bruises
- Sprains
Warnings
WARNINGS: EXTERNAL USE ONLY
DO NOT USE
- on wounds or damaged skin
- with a heating pad
- with or at the same times as other external analgesic
- if you are allergic to any ingredients of this product
When using this product
- do not use otherwise than as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
- discountinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
STOP USE and ask a doctor if
- rash, itching or excessive skin irritation develops
- conditions worsen
- symptoms persist for more than 7 days
- symptoms clear up and occur again with few days
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Propylene Glycol, Polysorbate 80, Carboxymethylcellulose Sodium, Carbomer, Purified Water, POVIDONE K90, Tartaric Acid, DMDM Hydantoin
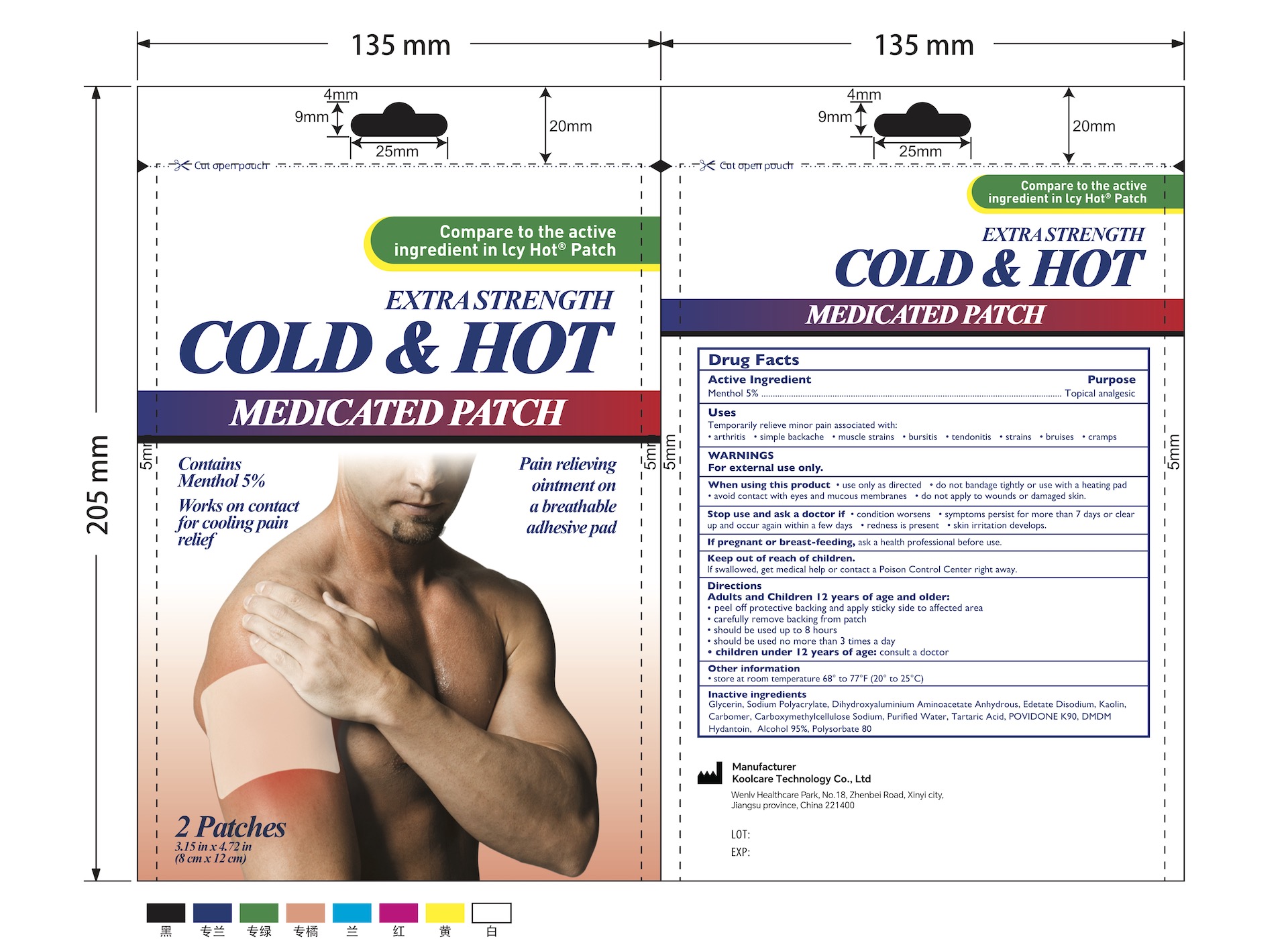
5% Menthol Pain Relief Patch (2 Patches/Pouch) NDC: 84205-006-00
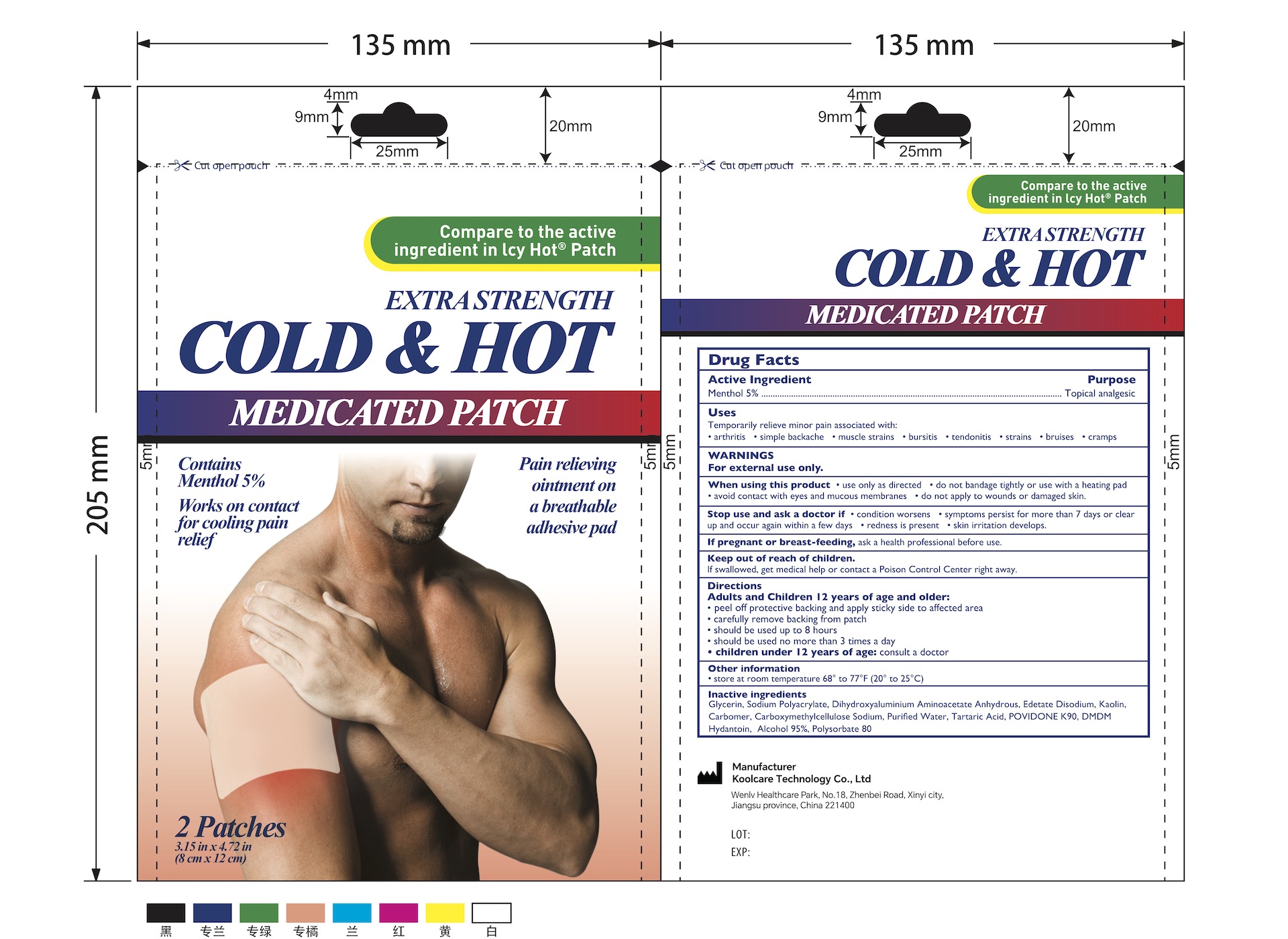
Uses
Topical Analgesic
Temporary relieve minor pain ssociated with:
- Arthritis
- Simple Backache
- Muscle Strains
- Bursitis
- Tendonitis
- Strains
- Bruises
- Cramps
Warnings
WARNINGS
For external use only.
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with the eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80
5% Menthol Pain Relief Patch (24 Pouches/Box) NDC: 84205-006-01

Uses
Topical Analgesic
Temporary relieve minor pain ssociated with:
- Arthritis
- Simple Backache
- Muscle Strains
- Bursitis
- Tendonitis
- Strains
- Bruises
- Cramps
Warnings
WARNINGS
For external use only.
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with the eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80
3% Menthol plus 0.083% Capsaicin Pain Relief Patch (2 Patches/Pouch) NDC: 84205-007-00
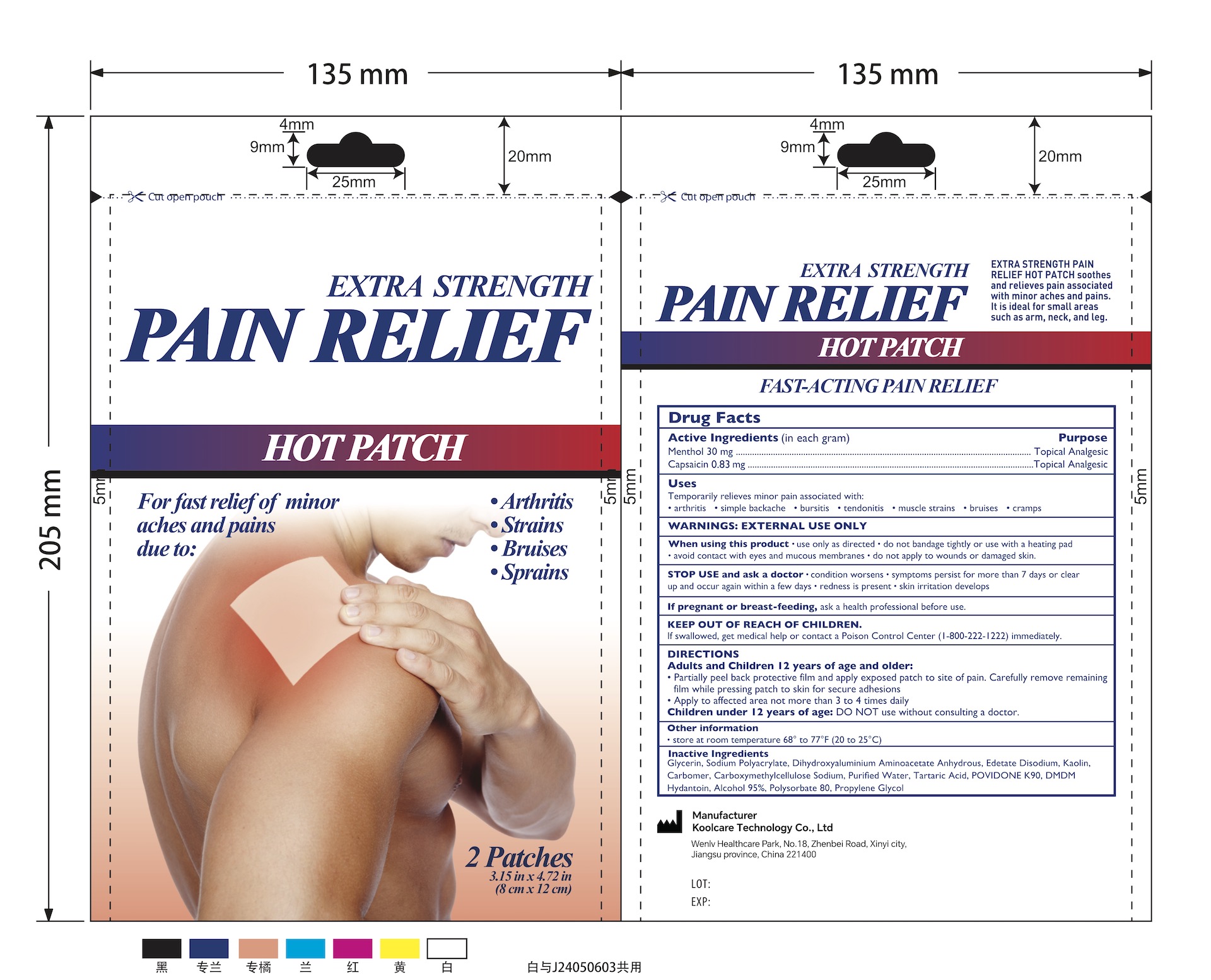
Uses
Topical Analgesic
Temporarily relieves minor pain ssociated with:
- Arthritis
- Simple Backache
- Bursitis
- Tendonitis
- Muscle Strains
- Bruises
- Cramps
Warnings
WARNINGS: EXTERNAL USE ONLY
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80, Propylene Glycol
Directions
DIRECTIONS
Adults and Children 12 years of age and older:
- Partially peel back protective film and apply exposed patch to site of pain. Carefully remove remaining film while pressing patch to skin for secure adhesions
- Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age: DO NOT use without consulting a doctor.
3% Menthol plus 0.083% Capsaicin Pain Relief Patch (24 Pouches/Box) NDC: 84205-007-01
Uses
Topical Analgesic
Temporarily relieves minor pain ssociated with:
- Arthritis
- Simple Backache
- Bursitis
- Tendonitis
- Muscle Strains
- Bruises
- Cramps
Warnings
WARNINGS: EXTERNAL USE ONLY
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops.
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80, Propylene Glycol
Directions
DIRECTIONS
Adults and Children 12 years of age and older:
- Partially peel back protective film and apply exposed patch to site of pain. Carefully remove remaining film while pressing patch to skin for secure adhesions
- Apply to affected area not more than 3 to 4 times daily
Children under 12 years of age: DO NOT use without consulting a doctor.
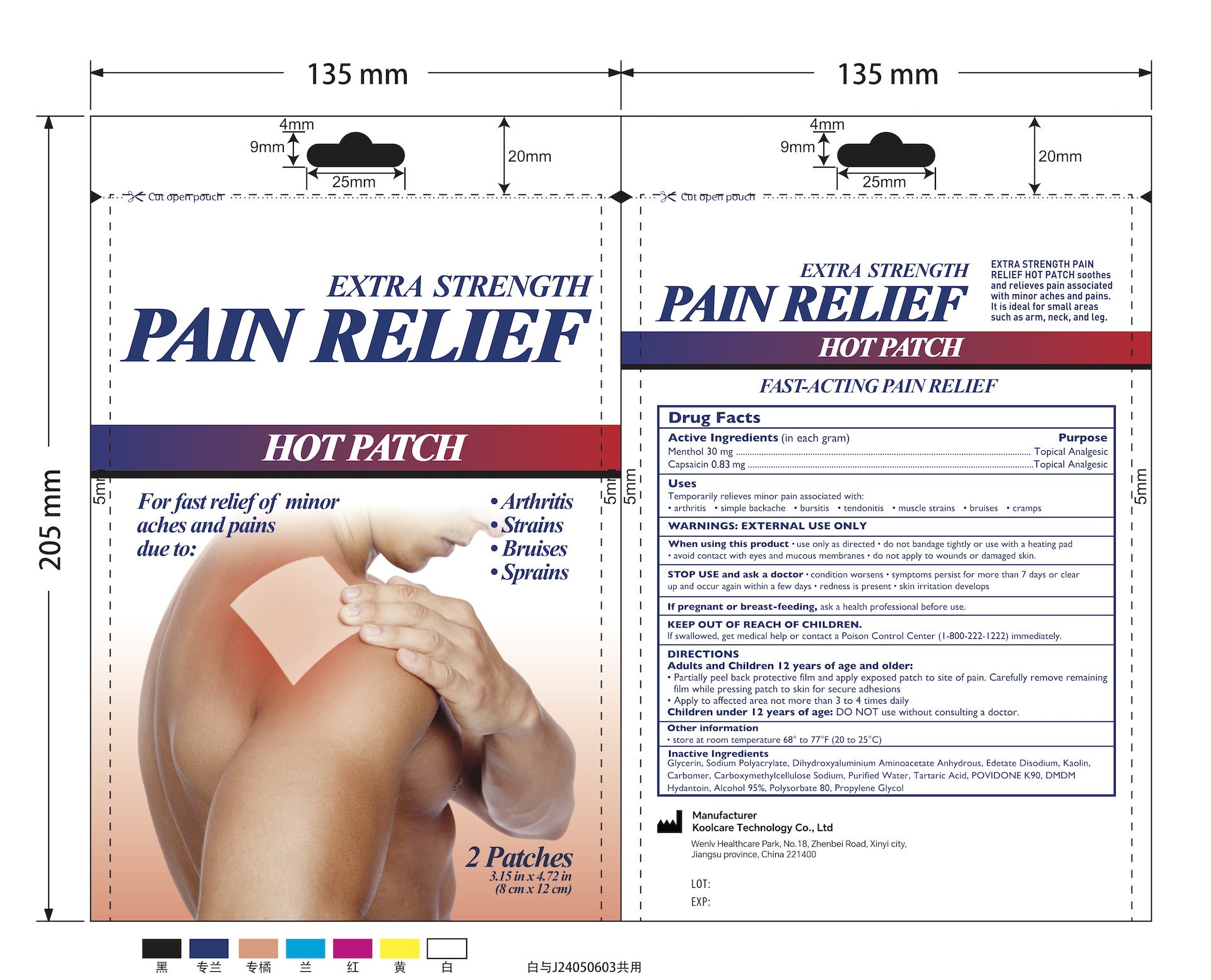
5% Menthol Pain Relief Patch (1 Large Patch/Pouch) NDC: 84205-008-00

Uses
Topical Analgesic
Temporarily relieve minor aches and pains of muscles and joints due to
- Simple Backache
- Arthritis
- Muscle Strains
- Bursitis
- Tendonitis
- Muscle Sprains
- Bruises
- Cramps
Warnings
WARNINGS: EXTERNAL USE ONLY
When using this product
- USE ONLY AS DIRECTED
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin.
STOP USE and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80
5% Menthol Pain Relief Patch (1 Large Patch X 24Patches) NDC: 84205-008-01

Uses
Topical Analgesic
Temporarily relieve minor aches and pains of muscles and joints due to
- Simple Backache
- Arthritis
- Muscle Strains
- Bursitis
- Tendonitis
- Muscle Sprains
- Bruises
- Cramps
Warnings
WARNINGS: EXTERNAL USE ONLY
When using this product
- USE ONLY AS DIRECTED
- do not bandage tightly or use with a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin.
STOP USE and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
- redness is present
- skin irritation develops
If pregnant or breast-feeding, ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN.
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) immediatedly.
Inactive Ingredients
Glycerin, Sodium Polyacrylate, Dihydroxyaluminium Aminoacetate Anhydrous, Edetate Disodium, Kaolin, Carbomer, Carboxymethylcellulose Sodium, Purified Water, Tartaric Acid, POVIDONE K90, DMDM Hydantoin, Alcohol 95%, Polysorbate 80
| 3% MENTHOL PLUS 0.083% CAPSAICIN PAIN RELIEF PATCH (2 PATCHES/POUCH)
pain relief patch, pain relief strip patch |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| 5% MENTHOL PAIN RELIEF PATCH (1 LARGE PATCH/POUCH)
pain relief patch patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| 5% MENTHOL PAIN RELIEF PATCH (2 PATCHES/POUCH)
pain relief patch patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| 0.025% CAPSAICIN PATCH (2 PATCHES/POUCH)
82632
capsaicin pain relief patch, capsaicin pain relief strip patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Koolcare Technology Co., Ltd (602479389) |
| Registrant - Koolcare Technology Co., Ltd (602479389) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Koolcare Technology Co., Ltd | 602479389 | manufacture(84205-005, 84205-006, 84205-007, 84205-008) | |