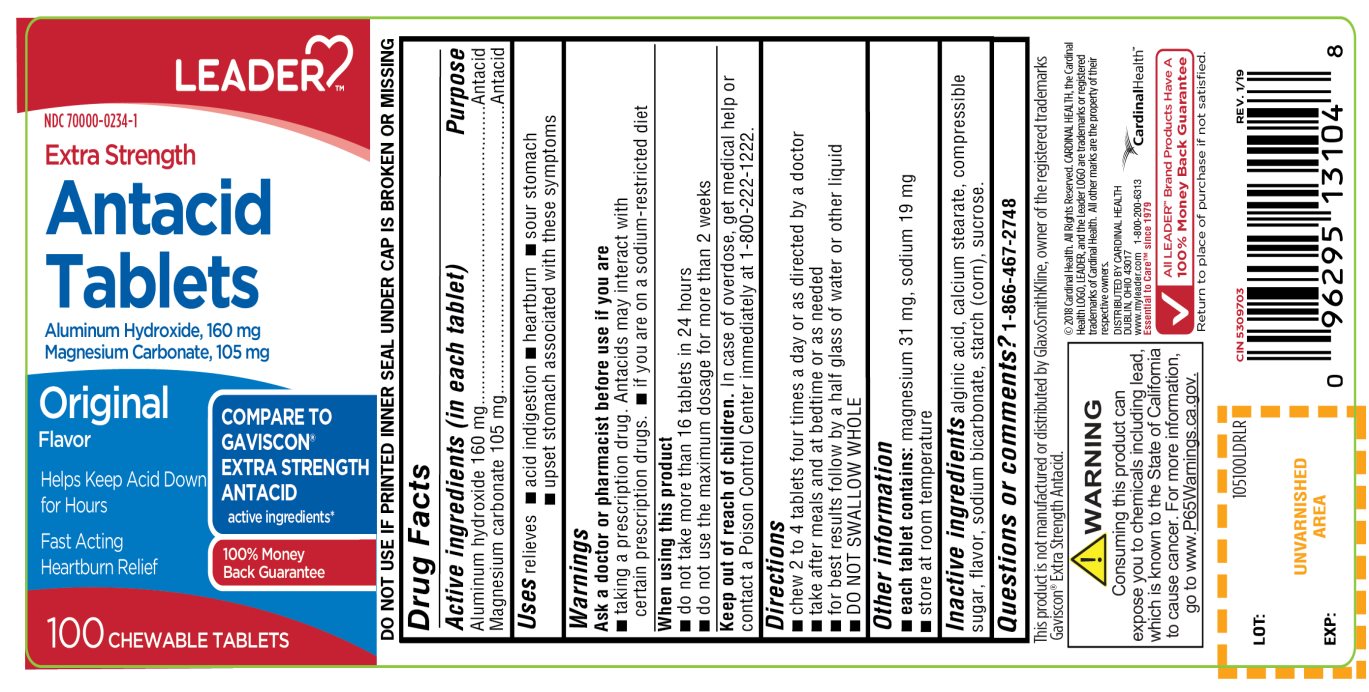
EXTRA STRENGTH ANTACID ORIGINAL FLAVOR- aluminum hydroxide and magnesium carbonate tablet, chewable
Extra Strength Antacid by
Drug Labeling and Warnings
Extra Strength Antacid by is a Otc medication manufactured, distributed, or labeled by Cardinal Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Consuming this product can expose you to chemicals including lead, which is known to the State of California to cause cancer. For more information, go to www.P65Warningd.ca.gov
Ask a doctor or pharmacist before use if you are
- taking a prescription drug. Antacids may interact with certain prescription drugs
- If you are on a sodium-restricted diet
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
LEADER™
NDC: 70000-0234-1
COMPARE TO GAVISCON® EXTRA STRENGTH ANTACID active ingredients*
Extra Strength
Antacid Tablets
Aluminum Hydroxide, 160 mg
Magnesium Carbonate, 105 mg
Original Flavor
- Helps Keep Acid Down for Hours
- Fast-Acting Heartburn Relief
100 Chewable Tablets
100% Money Back Guarantee
- DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING
©2018 Cardinal Health. All Right Reserved. CARDINAL HEALTH, the Cardinal Health LOGO, LEADER and the Leader LOGO are trademarks or registered
trademarks of Cardinal Health. All other marks are the property of their respective owners.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
All LEADER™ Brand Products Have A 100% Money Back Guarantee. Return to place of purchase if not satisfied.
This product is not manufactured or distributed by GlaxoSmithKline, owner of the registered trademarks of Gaviscon® Extra Strength Antacid.
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH ANTACID ORIGINAL FLAVOR
aluminum hydroxide and magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70000-0234 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) CORN SYRUP (UNII: 9G5L16BK6N) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 17mm Flavor BUTTERSCOTCH (ORIGINAL) Imprint Code RP105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70000-0234-1 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/04/2019 Labeler - Cardinal Health (097537435)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.