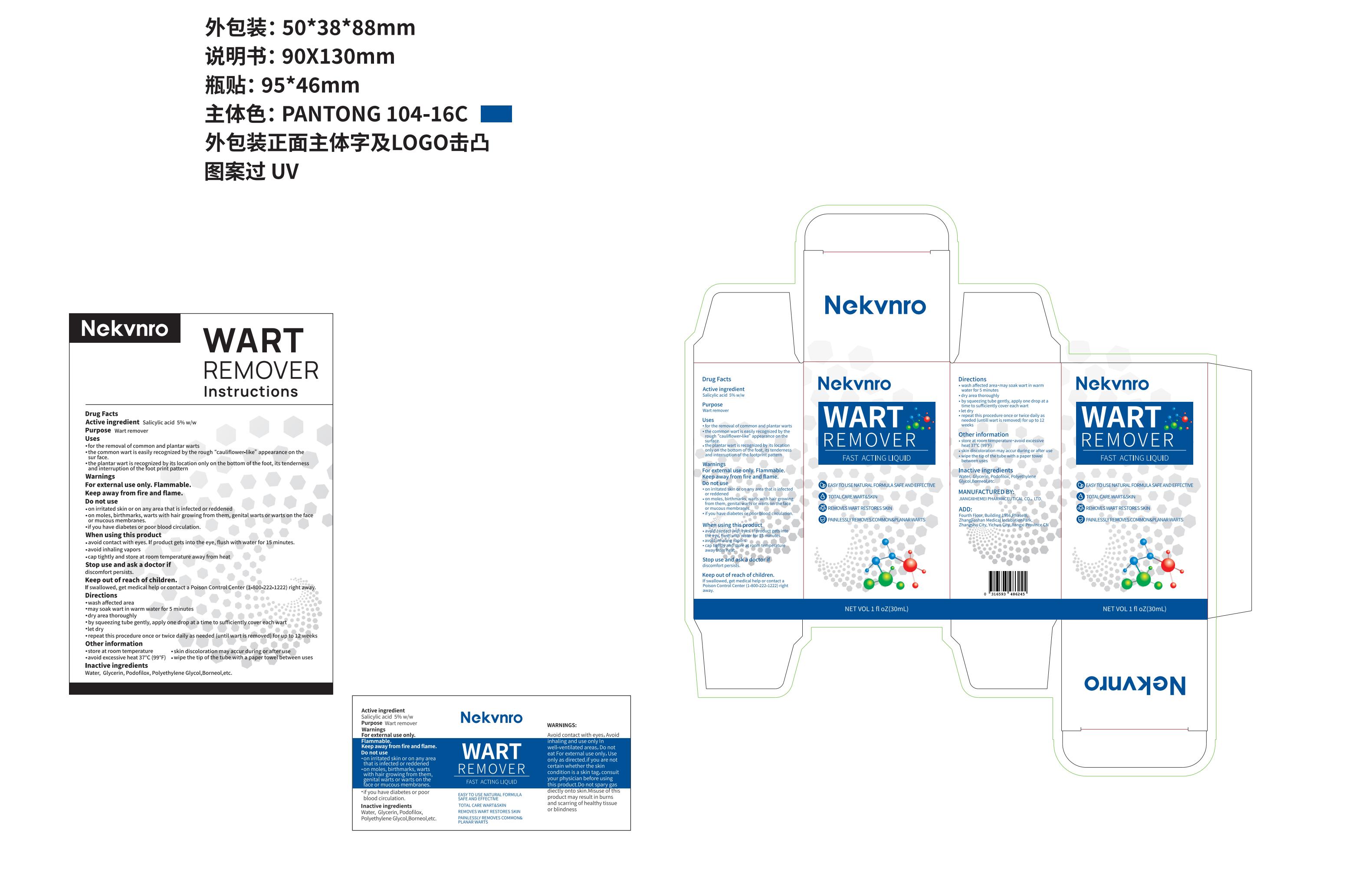
NeKvnro WART REMOVER by Jiangxi Hemei Pharmaceutical Co., Ltd 84010-024 complete
NeKvnro WART REMOVER by
Drug Labeling and Warnings
NeKvnro WART REMOVER by is a Otc medication manufactured, distributed, or labeled by Jiangxi Hemei Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEKVNRO WART REMOVER- salicylic acid wart remover liquid
Jiangxi Hemei Pharmaceutical Co., Ltd
----------
84010-024 complete
Use
·for the removal of common and plantar warts
·the common wart is easily recognized by the rough “cauliflower-like” appearance on the surface.
·the plantar wart is recognized by its location only on the bottom of the foot, its tenderness and interruption of the footprint pattern
Do not use
on irritated skin or on any area that is infected or reddened
·on moles, birthmarks, warts with hair growing from them, genital warts or warts on the face or mucous membranes.
·if you have diabetes or poor blood circulation.
When Using
·avoid contact with eyes.If product gets into the eye, flush with water for 15 minutes.
·avoid inhaling vapors
·cap tightly and store at room temperature away from heat
Keep Oot Of Reach Of Children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
wash affected area
·may soak wart in warm water for 5 minutes
·dry area thoroughly·by squeezing tube gently, apply one drop at a time to sufficiently cover each wart·let dry
·repeat this procedure once or twice daily as needed (until wart is removed) for up to 12 weeks
| NEKVNRO WART REMOVER
salicylic acid wart remover liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jiangxi Hemei Pharmaceutical Co., Ltd | 724892056 | manufacture(84010-024) | |