Ovide® (malathion) Lotion, 0.5%
OVIDE by
Drug Labeling and Warnings
OVIDE by is a Prescription medication manufactured, distributed, or labeled by TARO PHARMACEUTICALS USA, INC., TARO PHARMACEUTICAL INDUSTRIES LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OVIDE- malathion lotion
TARO PHARMACEUTICALS USA, INC.
----------
Ovide® (malathion) Lotion, 0.5%
DESCRIPTION
OVIDE Lotion contains 0.005 g of malathion per mL in a vehicle of isopropyl alcohol (78%), terpineol, dipentene, and pine needle oil. The chemical name of malathion is (±) - [(dimethoxyphosphinothioyl) - thio] butanedioic acid diethyl ester. Malathion has a molecular weight of 330.36, represented by C10H19O6PS2, and has the following chemical structure:
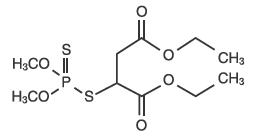
CLINICAL PHARMACOLOGY
Malathion is an organophosphate agent which acts as a pediculicide by inhibiting cholinesterase activity in vivo. Inadvertent transdermal absorption of malathion has occurred from its agricultural use. In such cases, acute toxicity was manifested by excessive cholinergic activity, i.e., increased sweating, salivary and gastric secretion, gastrointestinal and uterine motility, and bradycardia (see OVERDOSAGE). Because the potential for transdermal absorption of malathion from OVIDE Lotion is not known at this time, strict adherence to the dosing instructions regarding its use in children, method of application, duration of exposure, and frequency of application is required.
INDICATIONS AND USAGE
OVIDE Lotion is indicated for patients infected with Pediculus humanus capitis (head lice and their ova) of the scalp hair.
CONTRAINDICATIONS
OVIDE Lotion is contraindicated for neonates and infants because their scalps are more permeable and may have increased absorption of malathion. OVIDE Lotion should also not be used on individuals known to be sensitive to malathion or any of the ingredients in the vehicle.
WARNINGS
- OVIDE Lotion is flammable. The lotion and wet hair should not be exposed to open flames or electric heat sources, including hair dryers and electric curlers. Do not smoke while applying lotion or while hair is wet.
Allow hair to dry naturally and to remain uncovered after application of OVIDE Lotion. - OVIDE Lotion should only be used on children under the direct supervision of an adult.
- If OVIDE Lotion comes into contact with the eyes, flush immediately with water. Consult a physician if eye irritation persists.
- If skin irritation occurs, discontinue use of product until irritation clears. Reapply the OVIDE Lotion, and if irritation reoccurs, consult a physician.
- Chemical burns including second-degree burns and stinging sensations may occur with the use of OVIDE Lotion.
General
Keep out of reach of children. Close eyes tightly during product application. If accidentally placed in the eye, flush immediately with water. Use only on scalp hair.
Information to Patients
- OVIDE Lotion is flammable. The lotion and hair wet with lotion should not be exposed to open flames or electric heat sources, including hair dryers and electric curlers. Do not smoke while applying lotion or while hair is wet. The person applying OVIDE Lotion should wash hands after application. Allow hair to dry naturally and to remain uncovered after application of OVIDE Lotion.
- OVIDE Lotion should only be used on children under the direct supervision of an adult. Children should be warned to stay away from lighted cigarettes, open flames, and electric heat sources while the hair is wet.
- In case of accidental ingestion of OVIDE Lotion by mouth, seek medical attention immediately.
- If you are pregnant or nursing, you should contact your physician before using OVIDE Lotion.
- If OVIDE Lotion comes into contact with the eyes, flush immediately with water. Consult a physician if eye irritation persists or if visual changes occur.
- If skin irritation occurs, wash scalp and hair immediately. If the irritation clears, OVIDE Lotion may be reapplied. If irritation reoccurs, consult a physician.
- Burns and stinging sensations may occur when using OVIDE Lotion.
- Apply OVIDE Lotion on the scalp hair in an amount just sufficient to thoroughly wet hair and scalp. Pay particular attention to the back of the head and neck when applying OVIDE Lotion. Anyone applying OVIDE Lotion should wash hands immediately after the application process is complete.
- Allow hair to dry naturally and to remain uncovered. Shampoo hair after 8 to 12 hours, again paying attention to the back of the head and neck while shampooing.
- Rinse hair and use a fine - toothed (nit) comb to remove dead lice and eggs.
- If lice are still present after 7 - 9 days, repeat with a second application of OVIDE Lotion.
- Further treatment is generally not necessary. Other family members should be evaluated by a physician to determine if infested, and if so, receive treatment.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis, mutagenesis and impairment of fertility have not been studied with OVIDE Lotion (0.5% pharmaceutical grade malathion). However, following long-term oral administration of technical grade malathion to rodents via dietary supplementation, increased incidences of hepatocellular neoplastic lesions were observed in B6C3F1 mice dosed for 18 months at malathion doses greater than 1500 mg/ kg/day, and in female F344 rats dosed for 2 years at malathion doses greater than 400 mg/kg/day. These tumors occurred only in association with severe hepatic toxicity and chronic suppression of acetylcholinesterase activity, or at doses causing excessive mortality. Based on body surface area, doses at which carcinogenic effects were observed in rodents following life-time exposures to malathion were approximately 14- to 26-fold greater than the maximum dose anticipated in a 10 kg child following a single use of OVIDE Lotion, assuming 100% bioavailability. Actual systemic exposures are expected to be less than 10% of the administered dose.
The malathion of greater than pharmaceutical-grade purity used in OVIDE Lotion has not been tested for genotoxicity. The technical-grade malathion (95% pure) was found to be negative in Salmonella typhimurium, equivocally positive in the mouse lymphoma cell assay, and positive in in vitro chromosomal aberration and 425 sister chromatid exchange assays. Fifteen separate in vitro gene mutation studies with malathion of unknown purity have reported negative results, while three studies reported malathion to be mutagenic in bacterial cells. Both technical grade (94–96.5%) and purified (98-99%) malathion have been reported to cause chromosomal aberrations and sister chromatid exchanges in vitro in human and hamster cell lines. In vivo chromosomal aberration and micronucleus studies of technical-grade malathion are reported to be positive, whereas an in vivo chromosomal aberration study of >99% pure malathion was reported to be negative. Furthermore, mice exposed to malathion in their drinking water for 7 weeks demonstrated no evidence of chromosome damage in bone marrow cells, spermatogonia, or primary spermatocytes. Lack of details makes independent evaluation of the results of these assays impossible. Ashby and Purchase have suggested that impurities may be responsible for some of the observed genetic activity of malathion.
Reproduction studies performed with malathion in rats at doses over 180 fold greater than those anticipated in a 60 kg adult (based on body surface area and assuming 100% bioavailability) revealed no evidence of impaired fertility.
Pregnancy
Pregnancy Category B
There was no evidence of teratogenicity in studies in rats and rabbits at doses up to 900 mg/kg/day and 100 mg/kg/day malathion, respectively. A study in rats failed to show any gross fetal abnormalities attributable to feeding malathion up to 2,500 ppm (~ 200 mg/kg/day) in the diet during a three - generation evaluation period. These doses were approximately 40 to 180 times higher than the dose anticipated in a 60 kg adult (based on body surface area and assuming 100% bioavailability). Because animal reproduction studies are not always predictive of human responses, this drug should be used (or handled) during pregnancy only if clearly needed.
Nursing Mothers
Malathion in an acetone vehicle has been reported to be absorbed through human skin to the extent of 8% of the applied dose. However, percutaneous absorption from the OVIDE® (malathion) Lotion, 0.5% formulation has not been studied, and it is not known whether malathion is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when OVIDE Lotion is administered to (or handled by) a nursing mother.
ADVERSE REACTIONS
Malathion has been shown to be irritating to the skin and scalp. Other adverse reactions reported are chemical burns including second-degree burns. Accidental contact with the eyes can result in mild conjunctivitis.
It is not known if OVIDE Lotion has the potential to cause contact allergic sensitization.
OVERDOSAGE
Consideration should be given, as part of the treatment program, to the high concentration of isopropyl alcohol in the vehicle.
Malathion, although a weaker cholinesterase inhibitor than some other organophosphates, may be expected to exhibit the same symptoms of cholinesterase depletion after accidental ingestion orally. If accidentally swallowed, vomiting should be induced promptly or the stomach lavaged with 5% sodium bicarbonate solution.
Severe respiratory distress is the major and most serious symptom of organophosphate poisoning requiring artificial respiration, and atropine may be needed to counteract the symptoms of cholinesterase depletion.
Repeat analyses of serum and RBC cholinesterase may assist in establishing the diagnosis and formulating a long - range prognosis.
DOSAGE AND ADMINISTRATION
- Apply OVIDE Lotion on DRY hair in amount just sufficient to thoroughly wet the hair and scalp. Pay particular attention to the back of the head and neck while applying OVIDE Lotion. Wash hands after applying to scalp.
- Allow hair to dry naturally - use no electric heat source, and allow hair to remain uncovered.
- After 8 to 12 hours, the hair should be shampooed.
- Rinse and use a fine - toothed (nit) comb to remove dead lice and eggs.
- If lice are still present after 7 - 9 days, repeat with a second application of OVIDE Lotion.
Further treatment is generally not necessary. Other family members should be evaluated by a physician to determine if infested, and if so, receive treatment.
Clinical Studies
Two controlled clinical trials evaluated the pediculicidal activity of OVIDE Lotion. Patients applied the lotion to the hair and scalp in quantities, up to a maximum of 2 fl. oz., sufficient to thoroughly wet the hair and scalp. The lotion was allowed to air dry and was shampooed with Prell shampoo 8 to 12 hours after application. Patients in both the OVIDE Lotion group and in the vehicle group were examined immediately after shampooing, 24 hours after, and 7 days after for the presence of live lice. Results are shown in the following table:
| Treatment | Immediately After | 24 Hrs. After | 7 Days After |
|---|---|---|---|
| OVIDE Lotion | 129/129 | 122/129 | 114/126 |
| OVIDE Vehicle | 105/105 | 63/105 | 31/105 |
The presence or absence of ova at day 7 was not evaluated in these studies. The presence or absence of live lice or ova at 14 days following treatment was not evaluated in these studies. The residual amount of malathion on hair and scalp is unknown.
HOW SUPPLIED
OVIDE® (malathion) Lotion, 0.5%, is supplied in bottles of 2 fl. oz. (59 mL) NDC: 51672-5276-4.
Manufactured for:
TaroPharma®
a division of Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Manufactured by: Taro Pharmaceutical Industries Ltd., Haifa Bay, Israel, 26110
Ovide® and TaroPharma® are registered trademarks of Taro Pharmaceuticals U.S.A., Inc. and/or its affiliates.
Revised: November, 2011
70205--1111-1 425
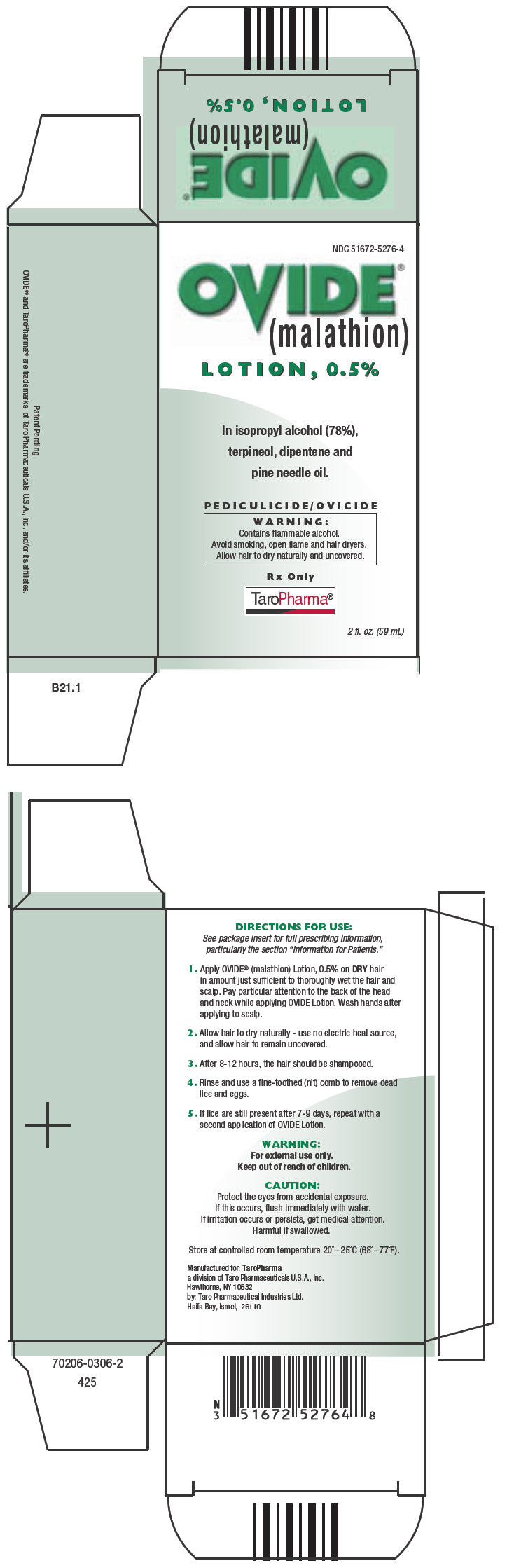
PRINCIPAL DISPLAY PANEL - 59 mL Carton
NDC: 51672-5276-4
OVIDE®
(malathion)
LOTION, 0.5%
In isopropyl alcohol (78%),
terpineol, dipentene and
pine needle oil.
PEDICULICIDE/OVICIDE
WARNING:
Contains flammable alcohol.
Avoid smoking, open flame and hair dryers.
Allow hair to dry naturally and uncovered.
Rx Only
TaroPharma®
2 fl. oz. (59 mL)
| OVIDE
malathion lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TARO PHARMACEUTICALS USA, INC. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TARO PHARMACEUTICAL INDUSTRIES LTD. | 600072078 | MANUFACTURE(51672-5276) | |
Trademark Results [OVIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OVIDE 73789557 1583279 Live/Registered |
GENDERM CORPORATION 1989-03-27 |
 OVIDE 73460314 1374592 Dead/Cancelled |
SYSECA-TEMPS REEL 1984-01-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.