PHYTONADIONE injection, emulsion PHYTONADIONE injection, emulsion
Phytonadione by
Drug Labeling and Warnings
Phytonadione by is a Prescription medication manufactured, distributed, or labeled by Teligent Pharma, Inc., Teligent, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PHYTONADIONE safely and effectively. See full prescribing information for PHYTONADIONE.
PHYTONADIONE injection, for intravenous, intramuscular, and subcutaneous use.
Initial U.S. Approval: 1960
WARNING – HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
See full prescribing information for complete boxed warning.
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after INTRAVENOUS and INTRAMUSCULAR injection of Phytonadione. Reactions have occurred despite dilution to avoid rapid infusion and upon first and subsequent doses. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified. (5.1)
RECENT MAJOR CHANGES
Warnings and Precautions, Cutaneous Reactions (5.3) 03/2018
INDICATIONS AND USAGE
Phytonadione is indicated for the treatment of the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity.
- Anticoagulant-induced hypoprothrombinemia deficiency caused by coumarin or indanedione derivatives; (1.1)
- Hypoprothrombinemia due to antibacterial therapy; (1.1)
- Hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis; (1.1)
- Other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism, e.g., salicylates. (1.1)
Phytonadione is indicated for prophylaxis and treatment of vitamin K-deficiency bleeding in neonates. (1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 2 mg/mL and 10 mg/mL single-dose ampuls. (3)
CONTRAINDICATIONS
Hypersensitivity to any component of this medication. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- Most common adverse reactions are cyanosis, diaphoresis, dizziness, dysgeusia, dyspnea, flushing, hypotension and tachycardia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teligent Pharma, Inc. at 1-856-697-1441, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Anticoagulants: May induce temporary resistance to prothrombin-depressing anticoagulants. (7)
USE IN SPECIFIC POPULATIONS
Pregnancy: If available, use the preservative-free formulation in pregnant women. (8.1)
Lactation: If available, use the preservative-free formulation in lactating women. (8.2)
Pediatric Use: The safety and effectiveness of Phytonadione in pediatric patients from 6 months to 17 years have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING - HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
1 INDICATIONS AND USAGE
1.1 Treatment of Hypoprothrombinemia Due to Vitamin K Deficiency or Interference
1.2 Prophylaxis and Treatment of Vitamin K Deficiency Bleeding in Neonates
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Considerations
2.2 Recommended Dosage for Coagulation Disorders from Vitamin K Deficiency or Interference
2.3 Recommended Dosage for Prophylaxis and Therapy of Vitamin K Deficiency Bleeding in Neonates
2.4 Directions for Dilution
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Risk of Serious Adverse Reaction in Infants due to Benzyl Alcohol Preservative
5.3 Cutaneous Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials and Post Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY SECTION
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING - HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE
Fatal hypersensitivity reactions, including anaphylaxis, have occurred during and immediately after intravenous and intramuscular injection of Phytonadione. Reactions have occurred despite dilution to avoid rapid intravenous infusion and upon first dose. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified [see WARNINGS AND PRECAUTIONS (5.1)].
-
1 INDICATIONS AND USAGE
1.1 Treatment of Hypoprothrombinemia Due to Vitamin K Deficiency or Interference
Phytonadione is indicated for the treatment of the following coagulation disorders which are due to faulty formation of factors II, VII, IX and X when caused by vitamin K deficiency or interference with vitamin K activity:
- anticoagulant-induced hypoprothrombinemia caused by coumarin or indanedione derivatives;
- hypoprothrombinemia due to antibacterial therapy;
- hypoprothrombinemia secondary to factors limiting absorption or synthesis of vitamin K, e.g., obstructive jaundice, biliary fistula, sprue, ulcerative colitis, celiac disease, intestinal resection, cystic fibrosis of the pancreas, and regional enteritis;
- other drug-induced hypoprothrombinemia where it is definitely shown that the result is due to interference with vitamin K metabolism, e.g., salicylates.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Considerations
Whenever possible, administer Phytonadione by the subcutaneous route [see WARNING — HYPERSENSITIVITY REACTIONS WITH INTRAVENOUS AND INTRAMUSCULAR USE]. When intravenous administration is unavoidable, inject the drug very slowly, not exceeding 1 mg per minute [see WARNINGS AND PRECAUTIONS (5.1)].
Monitor international normalized ratio (INR) regularly and as clinical conditions indicate. Use the lowest effective dose of Phytonadione.
The coagulant effects of Phytonadione are not immediate; improvement of INR may take 1-8 hours. Interim use of whole blood or component therapy may also be necessary if bleeding is severe.
Whenever possible, administer benzyl alcohol-free formulations in pediatric patients [see WARNINGS AND PRECAUTIONS(5.2) and USE IN SPECIFIC POPULATIONS (8.4)].
When Phytonadione is used to correct excessive anticoagulant-induced hypoprothrombinemia, anticoagulant therapy still being indicated, the patient is again faced with the clotting hazards existing prior to starting the anticoagulant therapy. Phytonadione is not a clotting agent, but overzealous therapy with Phytonadione may restore conditions which originally permitted thromboembolic phenomena. Dosage should be kept as low as possible, and INR should be checked regularly as clinical conditions indicate.
2.2 Recommended Dosage for Coagulation Disorders from Vitamin K Deficiency or Interference
The recommended dosage of Phytonadione is based on whether the hypoprothrombinemia is anticoagulant-induced (e.g., due to coumarin or indanedione derivatives) or non-anticoagulant-induced (e.g., due to antibiotics; salicylates or other drugs; factors limiting absorption or synthesis) as follows:
- Anticoagulant-Induced Hypoprothrombinemia: Phytonadione 2.5 mg to 10 mg or more subcutaneously, intramuscularly, or intravenously. Up to 25 mg to 50 mg may be administered as a single dose.
Repeated large doses of Phytonadione are not warranted in liver disease if the initial response is unsatisfactory. Failure to respond to Phytonadione may indicate that the condition being treated is inherently unresponsive to Phytonadione.
- Hypoprothrombinemia Due to Other Causes(Non-Anticoagulation-Induced Hypoprothrombinemia): Phytonadione 2.5 mg to 25 mg or more intravenously, intramuscularly, or subcutaneously. Up to 50 mg may be administered as a single dose.
Evaluate INR after 6-8 hours, and repeat dose if INR remains prolonged. Modify subsequent dosage (amount and frequency) based on the INR or clinical condition.
2.3 Recommended Dosage for Prophylaxis and Therapy of Vitamin K Deficiency Bleeding in Neonates
Prophylaxis of Vitamin K-Deficiency Bleeding in Neonates
The recommended dosage of Phytonadione is 0.5 mg to 1 mg within one hour of birth for a single dose.
Treatment of Vitamin K Deficiency Bleeding in Neonates
The recommended dosage of Phytonadione is 1 mg given either subcutaneously or intramuscularly. Consider higher doses if the mother has been receiving oral anticoagulants.
A failure to respond (shortening of the INR in 2 to 4 hours) may indicated another diagnosis or coagulation disorder.2.4 Directions for Dilution
Dilute Phytonadione with 0.9% Sodium Chloride Injection, 5% Dextrose Injection, or 5% Dextrose and Sodium Chloride Injection. Avoid use of other diluents that may contain benzyl alcohol, which can cause serious toxicity in newborns or low birth weight infants [see WARNINGS AND PRECAUTIONS (5.2) and USE IN SPECIFIC POPULATIONS (8.4)].
When diluted start administration of Phytonadione immediately after dilution.
Discard unused portions of diluted solution as well as unused contents of the ampul.
Protect Phytonadione from light at all times.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Hypersensitivity to phytonadione or any other component of this medication. [see WARNINGS AND PRECAUTIONS (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Fatal and severe hypersensitivity reactions, including anaphylaxis, have occurred with intravenous or intramuscular administration of Phytonadione. Reactions have occurred despite dilution to avoid rapid intravenous infusion and upon first dose. These reactions have included shock, cardiorespiratory arrest, flushing, diaphoresis, chest pain, tachycardia, cyanosis, weakness, and dyspnea. Administer Phytonadione subcutaneously whenever feasible. Avoid the intravenous and intramuscular routes of administration unless the subcutaneous route is not feasible and the serious risk is justified [see DOSAGE AND ADMINISTRATION (2)].
5.2 Risk of Serious Adverse Reaction in Infants due to Benzyl Alcohol Preservative
Use benzyl alcohol-free formulations in neonates and infants, if available. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and infants treated with benzyl alcohol-preserved drugs, including Phytonadione. The “gasping syndrome” is characterized by central nervous system depression, metabolic acidosis, and gasping respirations.
When prescribing Phytonadione in infants, consider the combined daily metabolic load of benzyl alcohol from all sources including Phytonadione (contains 9 mg of benzyl alcohol per mL) and other drugs containing benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see USE IN SPECIFIC POPULATIONS (8.1, 8.2 and 8.4)].
5.3 Cutaneous Reactions
Parenteral administration of vitamin K replacements (including Phytonadione) may cause cutaneous reactions. Reactions have included eczematous reactions, scleroderma-like patches, urticaria, and delayed-type hypersensitivity reactions. Time of onset ranged from 1 day to a year after parenteral administration. Discontinue Phytonadione for skin reactions and institute medical management.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see WARNINGS AND PRECAUTIONS (5.1)]
- Cutaneous Reactions [see WARNINGS AND PRECAUTIONS (5.3)]
6.1 Clinical Trials and Post Marketing Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions have been identified during post-approval use of Phytonadione. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: Tachycardia, hypotension
General disorders and administration site conditions: Generalized flushing pain, swelling and tenderness at injection site.
Hepatobiliary Disorders: Hyperbilirubinemia
Immune System Disorders: Fatal hypersensitivity reactions, anaphylactic reactions.
Neurologic: Dysgeusia, dizziness
Pulmonary: Dyspnea
Skin and Subcutaneous: Tissue Disorders: Erythema, pruritic plaques, scleroderma-like-lesions, erythema perstans.
Vascular: Cyanosis. -
7 DRUG INTERACTIONS
Anticoagulants
Phytonadione may induce temporary resistance to prothrombin-depressing anticoagulants, especially when larger doses of Phytonadione are used. Should this occur, higher doses of anticoagulant therapy may be needed when resuming anticoagulant therapy, or a change in therapy to a different class of anticoagulant may be necessary (i.e., heparin sodium).
Phytonadione does not affect the anticoagulant action of heparin. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Phytonadione contains benzyl alcohol, which has been associated with gasping syndrome in neonates. The preservative benzyl alcohol can cause serious adverse events and death when administered intravenously to neonates and infants. If Phytonadione is needed during pregnancy, consider using a benzyl alcohol-free formulation [see WARNINGS AND PRECAUTIONS (5.2) and USE IN SPECIFIC POPULATIONS (8.4)].
Published studies with the use of phytonadione during pregnancy have not reported a clear association with phytonadione and adverse developmental outcomes (see Data). There are maternal and fetal risks associated with vitamin K deficiency during pregnancy (see Clinical Considerations). Animal reproduction studies have not been conducted with phytonadione.
The estimated background risk for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnant women with vitamin K deficiency hypoprothrombinemia may be at an increased risk for bleeding diatheses during pregnancy and hemorrhagic events at delivery. Subclinical maternal vitamin K deficiency during pregnancy has been implicated in rare cases of fetal intracranial hemorrhage.Data
Human Data
Phytonadione has been measured in cord blood of infants whose mothers were treated with phytonadione during pregnancy in concentrations lower than seen in maternal plasma. Administration of vitamin K1 to pregnant women shortly before delivery increased both maternal and cord blood concentrations. Published data do not report a clear association with phytonadione and adverse maternal or fetal outcomes when used during pregnancy. However, these studies cannot definitively establish the absence of any risk because of methodologic limitations including small sample size and lack of blinding.Animal Data
In pregnant rats receiving vitamin K1 orally, fetal plasma and liver concentrations increased following administration, supporting placental transfer.8.2 Lactation
Risk Summary
Phytonadione contains benzyl alcohol. If available, preservative-free Phytonadione is recommended when Phytonadione is needed during lactation [see WARNINGS AND PRECAUTIONS (5.2) and USE IN SPECIFIC POPULATIONS (8.4)].
Phytonadione is present in breastmilk. There are no data on the effects of Phytonadione on the breastfed child or on milk production. The developmental and health benefits of breastfeeding should be considered along with the clinical need for Phytonadione and any potential adverse effects on the breastfed child from Phytonadione or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of Phytonadione for prophylaxis and treatment of vitamin K deficiency have been established in neonates. Use of phytonadione injection for prophylaxis and treatment of vitamin K deficiency is based on published clinical studies.
Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and infants in the intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol.
When prescribing Phytonadione in infants consider the combined daily metabolic load of benzyl alcohol from all sources including Phytonadione (Phytonadione contains 9 mg of benzyl alcohol) and other drugs containing benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see WARNINGS AND PRECAUTIONS (5.2)].
Whenever possible, use preservative-free phytonadione formulations in neonates. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients. Premature and low-birth weight infants may be more likely to develop toxicity.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Phytonadione is a vitamin K replacement, which is a clear, yellow to amber, viscous, odorless or nearly odorless liquid. It is insoluble in water, soluble in chloroform and slightly soluble in ethanol. It has a molecular weight of 450.70.
Phytonadione is 2-methyl-3-phytyl-1, 4-naphthoquinone. Its empirical formula is C31H46O2 and its molecular structure is:
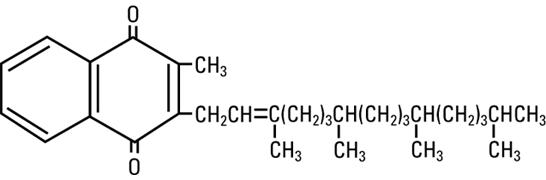
Phytonadione injection is a yellow, sterile, aqueous colloidal solution of vitamin K1, with a pH of 5.0 to 7.0, available for injection by the intravenous, intramuscular, and subcutaneous routes. Phytonadione is available in 1 mg (2 mg/mL) and 10 mg (10 mg/mL) single-dose ampuls. Each milliliter of Phytonadione contains the following inactive ingredients: 70 mg polyoxyethylated fatty acid derivative, 37.5 mg dextrose, 9 mg benzyl alcohol (preservative), and water for injection. Phytonadione contains glacial acetic acid for pH adjustment to 6.3 (5.0 – 7.0).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Phytonadione aqueous colloidal solution of vitamin K1 for parenteral injection, possesses the same type and degree of activity as does naturally-occurring vitamin K, which is necessary for the production via the liver of active prothrombin (factor II), proconvertin (factor VII), plasma thromboplastin component (factor IX), and Stuart factor (factor X). Vitamin K is an essential cofactor for a microsomal enzyme that catalyzes the post- translational carboxylation of multiple, specific, peptide-bound glutamic acid residues in inactive hepatic precursors of factors II, VII, IX, and X. The resulting gamma-carboxy-glutamic acid residues convert the precursors into active coagulation factors that are subsequently secreted by liver cells into the blood.
In normal animals and humans, phytonadione is virtually devoid of activity. However, in animals and humans deficient in vitamin K, the pharmacological action of vitamin K is related to its normal physiological function, that is, to promote the hepatic biosynthesis of vitamin K dependent clotting factors.12.2 Pharmacodynamics
The action of the aqueous dispersion, when administered intravenously, is generally detectable within an hour or two and hemorrhage is usually controlled within 3 to 6 hours. A normal INR may often be obtained in 12 to 14 hours.
12.3 Pharmacokinetics
Absorption:
Phytonadione is readily absorbed following intramuscular administration.
Distribution:
After absorption, phytonadione is initially concentrated in the liver, but the concentration declines rapidly. Very little vitamin K accumulates in tissues.
Elimination:
Little is known about the metabolic fate of vitamin K. Almost no free unmetabolized vitamin K appears in bile or urine. - 13 NONCLINICAL TOXICOLOGY SECTION
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Phytonadione is a yellow, sterile, aqueous colloidal solution and is supplied in a package of 25 as follows:
NDC No. Container Amount of
Phytonadione
In ContainerVolume Concentration 52565-142-25 1 mL single-dose ampul 1 mg 0.5 mL 2 mg/mL 52565-143-25 1 mL single-dose ampul 10 mg 1 mL 10 mg/mL Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Protect Phytonadione from light. Store container in closed original carton until contents have been used.
NOTE: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform the patient of the following important risks of Phytonadione.
Serious Hypersensitivity Reactions
Advise the patient and caregivers to immediately report signs of hypersensitivity after receiving Phytonadione [see WARNINGS AND PRECAUTIONS (5.1)].
Risk of Gasping Syndrome Due to Benzyl Alcohol
Advise the patient and caregivers of the risk of gasping syndrome associated with the use of products that contain benzyl alcohol (including Phytonadione) in neonates, infants, and pregnant women [see WARNINGS AND PRECAUTIONS (5.2)].
Cutaneous Reactions
Advise the patient and caregivers to report the occurrence of new rashes after receiving Phytonadione. These reactions may be delayed for up to a year after treatment [see WARNINGS AND PRECAUTIONS (5.3)].Manufactured by:
Teligent Pharma, Inc.
Buena, NJ 08310
-
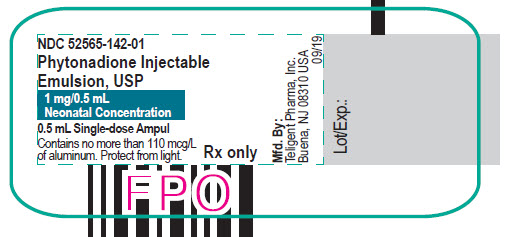
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
0.5 mL Single-dose Ampul
NDC: 52565-142-01
Phytonadione Injectable Emulsion, US
1 mg/0.5 mL
Neonatal Concentration
Contains no more than 110 mcg/L of aluminum.
Protect from light.
Rx only
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
1 mL Single-dose Ampul
NDC: 52565-143-01
Phytonadione Injectable Emulsion, USP
10 mg/mL
Contains no more than 110 mcg/L of aluminum.
Protect from light.
Rx only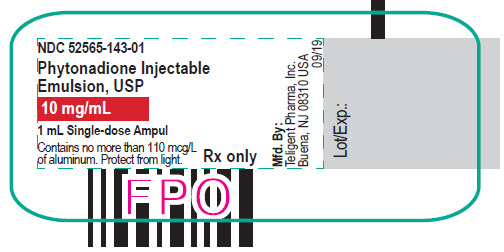
-
INGREDIENTS AND APPEARANCE
PHYTONADIONE
phytonadione injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52565-142 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) 70 mg in 1 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 37.5 mg in 1 mL WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) 9 mg in 1 mL Acetic Acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52565-142-25 25 in 1 TRAY 03/07/2018 1 0.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA012223 03/07/2018 PHYTONADIONE
phytonadione injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52565-143 Route of Administration INTRAMUSCULAR, INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHYTONADIONE (UNII: A034SE7857) (PHYTONADIONE - UNII:A034SE7857) PHYTONADIONE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) 70 mg in 1 mL DEXTROSE MONOHYDRATE (UNII: LX22YL083G) 37.5 mg in 1 mL WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) 9 mg in 1 mL Acetic Acid (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52565-143-25 5 in 1 TRAY 03/07/2018 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA012223 03/07/2018 Labeler - Teligent Pharma, Inc. (011036910) Registrant - Teligent Pharma, Inc. (011036910) Establishment Name Address ID/FEI Business Operations Teligent, Inc. 011036910 manufacture(52565-142, 52565-143)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.