THIOLA EC- tiopronin tablet, delayed release
Thiola EC by
Drug Labeling and Warnings
Thiola EC by is a Prescription medication manufactured, distributed, or labeled by Mission Pharmacal Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use THIOLA® EC safely and effectively. See full prescribing information for THIOLA EC.
THIOLA EC (tiopronin) delayed-release tablets, for oral use
Initial U.S. Approval: 1988INDICATIONS AND USAGE
THIOLA EC is a reducing and complexing thiol indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in adults and pediatric patients 20 kg and greater with severe homozygous cystinuria, who are not responsive to these measures alone. (1)
DOSAGE AND ADMINISTRATION
- The recommended initial dosage in adult patients is 800 mg/day. In clinical studies, the average dosage was about 1,000 mg/day. (2.1)
- The recommended initial dosage in pediatric patients 20 kg and greater is 15 mg/kg/day. Avoid dosages greater than 50 mg/kg per day in pediatric patients. (2.1, 5.1, 8.4)
- Administer THIOLA EC in 3 divided doses at the same times each day, with or without food. Maintain a routine pattern with regard to meals. (2.1)
- Swallow THIOLA EC tablets whole. (2.1)
- Measure urinary cystine 1 month after initiation of THIOLA EC and every 3 months thereafter. (2.1)
DOSAGE FORMS AND STRENGTHS
Tablets: 100 mg and 300 mg (3)
CONTRAINDICATIONS
- Hypersensitivity to tiopronin or any component of THIOLA EC (4)
WARNINGS AND PRECAUTIONS
- Proteinuria, including nephrotic syndrome, and membranous nephropathy, has been reported with tiopronin use. Pediatric patients receiving greater than 50 mg/kg of tiopronin per day may be at increased risk for proteinuria. (2.1, 5.1, 8.4)
- Hypersensitivity reactions have been reported during tiopronin treatment. (4, 5.2)
ADVERSE REACTIONS
Most common adverse reactions (≥10%) are nausea, diarrhea or soft stools, oral ulcers, rash, fatigue, fever, arthralgia, proteinuria, and emesis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mission Pharmacal Company at toll-free phone # 1-800-298-1087 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Proteinuria
5.2 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Alcohol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Adults: The recommended initial dosage in adult patients is 800 mg/day. In clinical studies, the average dosage was about 1,000 mg/day.
Pediatrics: The recommended initial dosage in pediatric patients weighing 20 kg and greater is 15 mg/kg/day. Avoid dosages greater than 50 mg/kg per day in pediatric patients [see Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
Administer THIOLA EC in 3 divided doses at the same times each day, with or without food. Maintain a routine pattern with regard to meals. Swallow THIOLA EC tablets whole.
Consider starting THIOLA EC at a lower dosage in patients with history of severe toxicity to d-penicillamine.
2.2 Monitoring
Measure urinary cystine 1 month after starting THIOLA EC and every 3 months thereafter. Adjust THIOLA EC dosage to maintain urinary cystine concentration less than 250 mg/L.
Assess for proteinuria before treatment and every 3 to 6 months during treatment [see Warnings and Precautions (5.1)].
Discontinue THIOLA EC in patients who develop proteinuria, and monitor urinary protein and renal function. Consider restarting THIOLA EC treatment at a lower dosage after resolution of proteinuria.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
THIOLA EC is contraindicated in patients with hypersensitivity to tiopronin or any other components of THIOLA EC [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Proteinuria
Proteinuria, including nephrotic syndrome, and membranous nephropathy, have been reported with tiopronin use. Pediatric patients receiving greater than 50 mg/kg of tiopronin per day may be at increased risk for proteinuria. [see Dosage and Administration (2.2), Adverse Reactions (6.1, 6.2) Use in Specific Populations (8.4)]. Monitor patients for the development of proteinuria and discontinue therapy in patients who develop proteinuria [see Dosage and Administration (2.2)].
5.2 Hypersensitivity Reactions
Hypersensitivity reactions (drug fever, rash, fever, arthralgia and lymphadenopathy) have been reported [see Contraindications (4)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Proteinuria [see Warnings and Precautions (5.1)]
- Hypersensitivity [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of the drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions occurring at an incidence of ≥5% in an uncontrolled trial in 66 patients with cystinuria age 9 to 68 years are shown in the table below. Patients in group 1 had previously been treated with d-penicillamine; those in group 2 had not. Of those patients who had stopped taking d-penicillamine due to toxicity (34 out of 49 patients in group 1), 22 were able to continue treatment with THIOLA. In those without prior history of d-penicillamine treatment, 6% developed reactions of sufficient severity to require THIOLA withdrawal.
Table 1 presents adverse reactions ≥5% in either treatment group occurring in this trial.
Table 1: Adverse Reactions Occurring in One or More Patients System Organ Class Adverse Reaction Group 1
Previously treated with
d‑penicillamine
(N = 49)Group 2
Naïve to d‑penicillamine
(N = 17)Blood and Lymphatic System Disorders anemia 1 (2%) 1 (6%) Gastrointestinal Disorders nausea 12 (25%) 2 (12%) emesis 5 (10%) – diarrhea/soft stools 9 (18%) 1 (6%) abdominal pain – 1 (6%) oral ulcers 6 (12%) 3 (18%) General Disorders and Administration Site Conditions fever 4 (8%) – weakness 2 (4%) 2 (12%) fatigue 7 (14%) – peripheral (edema) 3 (6%) 1 (6%) chest pain – 1 (6%) Metabolism and Nutrition Disorders anorexia 4 (8%) – Musculoskeletal and Connective Tissue Disorders arthralgia – 2 (12%) Renal and Urinary Disorders proteinuria 5 (10%) 1 (6%) impotence – 1 (6%) Respiratory, Thoracic and Mediastinal Disorders cough – 1 (6%) Skin and Subcutaneous Tissue Disorders rash 7 (14%) 2 (12%) ecchymosis 3 (6%) – pruritus 2 (4%) 1 (6%) urticaria 4 (8%) – skin wrinkling 3 (6%) 1 (6%) Taste Disturbance
A reduction in taste perception may develop. It is believed to be the result of chelation of trace metals by tiopronin. Hypogeusia is often self-limited.6.2 Postmarketing Experience
Adverse reactions have been reported from the literature, as well as during post-approval use of THIOLA. Because the post-approval reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to THIOLA exposure.
Adverse reactions reported during the postmarketing use of THIOLA are listed by body system in Table 2.
Table 2: Adverse Reactions Reported for THIOLA Pharmacovigilance by System Organ Class and Preferred Term System Organ Class Preferred Term Cardiac Disorders congestive heart failure Ear and Labyrinth Disorder vertigo Gastrointestinal Disorders abdominal discomfort; abdominal distension; abdominal pain; chapped lips; diarrhea; dry mouth; dyspepsia; eructation; flatulence; gastrointestinal disorder; gastroesophageal reflux disease; nausea; vomiting; jaundice; liver transaminitis General Disorders and Administration Site Conditions asthenia; chest pain; fatigue; malaise; pain; peripheral swelling; pyrexia; swelling Investigations glomerular filtration rate decreased; weight increased Metabolism and Nutrition Disorders decreased appetite; dehydration; hypophagia Musculoskeletal and Connective Tissue Disorders arthralgia; back pain; flank pain; joint swelling; limb discomfort; musculoskeletal discomfort; myalgia; neck pain; pain in extremity Nervous System Disorders ageusia; burning sensation; dizziness; dysgeusia; headache; hypoesthesia Renal and Urinary Disorders nephrotic syndrome; proteinuria; renal failure Skin and Subcutaneous Tissue Disorders dry skin; hyperhidrosis; pemphigus foliaceus; pruritus; rash; rash pruritic; skin irritation; skin texture abnormal; skin wrinkling; urticaria -
7 DRUG
INTERACTIONS
7.1 Alcohol
Tiopronin is released faster from THIOLA EC in the presence of alcohol and the risk for adverse events associated with THIOLA EC when taken with alcohol is unknown. Avoid alcohol consumption 2 hours before and 3 hours after taking THIOLA EC [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available published case report data with tiopronin have not identified a drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. Renal stones in pregnancy may result in adverse pregnancy outcomes (see Clinical Considerations). In animal reproduction studies, there were no adverse developmental outcomes with oral administration of tiopronin to pregnant mice and rats during organogenesis at doses up to 2 times a 2 grams/day human dose (based on mg/m2). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2% to 4% and 15% to 20%, respectively.Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Renal stones in pregnancy may increase the risk of adverse pregnancy outcomes, such as preterm birth and low birth weight.Data
Animal Data
No findings of fetal malformations could be attributed to the drug in reproduction studies in mice and rats at doses up to 2 times the highest recommended human dose of 2 grams/day (based on mg/m2).8.2 Lactation
Risk Summary
There are no data on the presence of tiopronin in either human or animal milk or on the effects of the breastfed child. A published study suggests that tiopronin may suppress milk production. Because of the potential for serious adverse reactions, including nephrotic syndrome, advise patients that breastfeeding is not recommended during treatment with THIOLA EC.8.4 Pediatric Use
THIOLA EC is indicated in pediatric patients weighing 20 kg or more with severe homozygous cystinuria, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation who are not responsive to these measures alone. This indication is based on safety and efficacy data from a trial in patients 9 years to 68 years of age and clinical experience. Proteinuria, including nephrotic syndrome, has been reported in pediatric patients. Pediatric patients receiving greater than 50 mg/kg tiopronin per day may be at greater risk [see Dosage and Administration (2.1, 2.2), Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
THIOLA EC tablets are not approved for use in pediatric patients weighing less than 20 kg or in pediatric patients unable to swallow tablets [see Dosage and Administration (2.1)].
8.5 Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- 10 OVERDOSAGE
-
11 DESCRIPTION
THIOLA EC (tiopronin) delayed-release tablets are a reducing and cystine-binding thiol drug (CBTD) for oral use. Tiopronin is N‑(2‑Mercaptopropionyl) glycine and has the following structure:

Tiopronin has the empirical formula C5H9NO3S and a molecular weight of 163.20. In this drug product tiopronin exists as a dl racemic mixture.
Tiopronin is a white crystalline powder, which is freely soluble in water.
Each THIOLA EC tablet contains 100 or 300 mg of tiopronin. The inactive ingredients in THIOLA EC tablets include lactose monohydrate, hydroxypropyl cellulose, hydroxypropyl cellulose (low substitute), magnesium stearate, hydroxypropyl methylcellulose E5, methacrylic acid: ethyl acrylate copolymer (Eudragit L 100-55), talc, triethyl citrate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The goal of therapy is to reduce urinary cystine concentration below its solubility limit. Tiopronin is an active reducing agent which undergoes thiol-disulfide exchange with cystine to form a mixed disulfide of tiopronin-cysteine. From this reaction, a water-soluble mixed disulfide is formed and the amount of sparingly soluble cystine is reduced.
12.2 Pharmacodynamics
The decrement in urinary cystine produced by tiopronin is generally proportional to the dose. A reduction in urinary cystine of 250-350 mg/day at tiopronin dosage of 1 g/day, and a decline of approximately 500 mg/day at a dosage of 2 g/day, might be expected. Tiopronin has a rapid onset and offset of action, showing a fall in cystine excretion on the first day of administration and a rise on the first day of drug withdrawal.
12.3 Pharmacokinetics
Absorption
THIOLA EC Tablets
When THIOLA IR and THIOLA EC single doses were given to fasted healthy subjects (n = 39) in a crossover study, the median time to peak plasma levels (Tmax) were 1 (range: 0.5 to 2.1) and 3 (range: 1.0 to 6.0) hours, respectively. The peak exposure (Cmax) and total exposure (AUC0-t) of tiopronin from THIOLA EC tablets were decreased by 22% and 7% respectively compared to THIOLA IR tablets.Food Effects
Administration of the THIOLA EC tablet with food decreases Cmax of tiopronin by 13% and AUC0-t by 25% compared to THIOLA EC administered in a fasted state. Since the drug is dosed to effect, the study results support administration of THIOLA EC tablets with or without food; administer at the same time each day with a routine pattern with regard to meals.Elimination
Excretion
When tiopronin is given orally, up to 48% of dose appears in urine during the first 4 hours and up to 78% by 72 hours.Drug Interactions
Alcohol
An in vitro dissolution study was conducted to evaluate the impact of alcohol (5, 10, 20, and 40%) on the dose dumping of THIOLA EC tablets. The study results showed that the addition of alcohol to the dissolution media increases the dissolution rate of THIOLA EC tablets in the acidic media of 0.1N HCl [see Drug Interactions (7.1)]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term carcinogenicity studies in animals have not been performed.Mutagenesis
Tiopronin was not genotoxic in the chromosomal aberration, sister chromatid exchange, and in vivo micronucleus assays.Impairment of Fertility
High doses of tiopronin in experimental animals have been shown to interfere with maintenance of pregnancy and viability of the fetus. In 2 published male fertility studies in rats, tiopronin at 20 mg/kg/day intramuscular (IM) for 60 days induced reductions in testis, epididymis, vas deferens, and accessory sex glands weights and in the count and motility of cauda epididymal sperm. -
16 HOW
SUPPLIED/STORAGE AND HANDLING
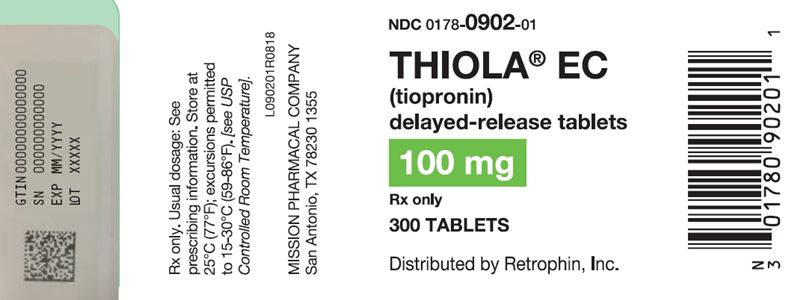
100 mg delayed-release, round, white to off-white tablet imprinted with “T1” on one side with red ink and blank on the other side: Bottles of 300 NDC 0178-0902-01.
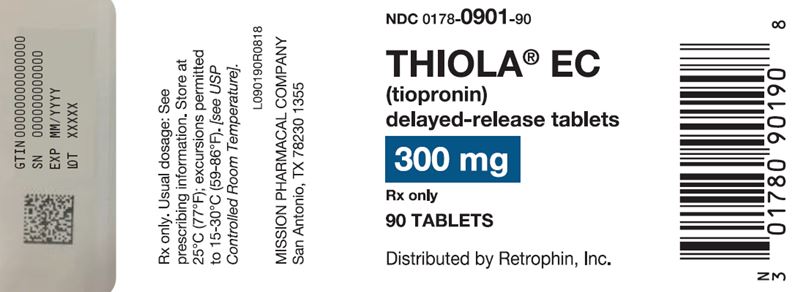
300 mg delayed-release, round, white to off-white tablet imprinted with “T3” on one side with red ink and blank on the other side: Bottles of 90 NDC 0178-0901-90.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Administration Instructions
Advise patients to swallow THIOLA EC tablets intact and not to chew, crush, or split the tablets.Lactation
Advise women that breastfeeding is not recommended during treatment with THIOLA EC [see Use in Specific Populations (8.2)].Manufactured and packaged by Mission Pharmacal Company, San Antonio, TX 78230 1355
Distributed by Retrophin, Inc., San Diego, CA 92130 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
THIOLA EC
tiopronin tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0178-0902 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIOPRONIN (UNII: C5W04GO61S) (TIOPRONIN - UNII:C5W04GO61S) TIOPRONIN 100 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TALC (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code T1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0178-0902-01 300 in 1 BOTTLE; Type 0: Not a Combination Product 06/28/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211843 06/28/2019 THIOLA EC
tiopronin tablet, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0178-0901 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIOPRONIN (UNII: C5W04GO61S) (TIOPRONIN - UNII:C5W04GO61S) TIOPRONIN 300 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) TALC (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 11mm Flavor Imprint Code T3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0178-0901-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/28/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211843 06/28/2019 Labeler - Mission Pharmacal Company (008117095) Registrant - Mission Pharmacal Company (927726893) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 MANUFACTURE(0178-0901, 0178-0902)
Trademark Results [Thiola EC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 THIOLA EC 88068538 5904582 Live/Registered |
Mission Pharmacal Company 2018-08-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.