Cetirizine HCl by TOP CARE (Topco Associates LLC) / Bionpharma Inc. / Patheon Softgels Inc. CETIRIZINE HCL capsule
Cetirizine HCl by
Drug Labeling and Warnings
Cetirizine HCl by is a Otc medication manufactured, distributed, or labeled by TOP CARE (Topco Associates LLC), Bionpharma Inc., Patheon Softgels Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
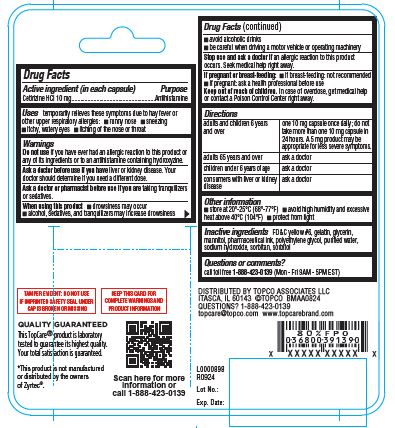
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg capsule once daily; do not take more than one 10 mg capsule in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
KEEP THIS CARD FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
QUALITY GUARANTEED
This TopCare® product is laboratory tested to guarantee its highest quality. Your total satisfaction is guaranteed.
†This product is not manufactured or distributed by the owners of Zyrtec ®.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ITASCA, IL 60143 ©TOPCO BMAA0824
QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.comL0000898
R0924
-
Principal Display Panel
NDC: 36800-542-86
TopCare®
health
COMPARE TO THE ACTIVE
INGREDIENT IN ZYRTEC®*All-Day
Allergy
CETIRIZINE HCI CAPSULES, 10mg
ANTIHISTAMINEIndoor & outdoor allergies
24-hourRELIEF OF:
Sneezing
Runny nose
Itchy, watery eyes
Itchy throat or noseactual size
25
SOFTGELS**
**LIQUID-FILLED CAPSULES

-
INGREDIENTS AND APPEARANCE
CETIRIZINE HCL
cetirizine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36800-542 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MANNITOL (UNII: 3OWL53L36A) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange Score no score Shape OVAL Size 13mm Flavor Imprint Code CE1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36800-542-86 1 in 1 PACKAGE 11/22/2024 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 36800-542-15 1 in 1 PACKAGE 11/22/2024 2 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022429 11/22/2024 Labeler - TOP CARE (Topco Associates LLC) (006935977) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Patheon Softgels Inc. 002193829 manufacture(36800-542)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.