IZKUT Fungal Nail Patches by Shenzhen Situya Trading Co., Ltd. 82739-022 Complete
IZKUT Fungal Nail Patches by
Drug Labeling and Warnings
IZKUT Fungal Nail Patches by is a Otc medication manufactured, distributed, or labeled by Shenzhen Situya Trading Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
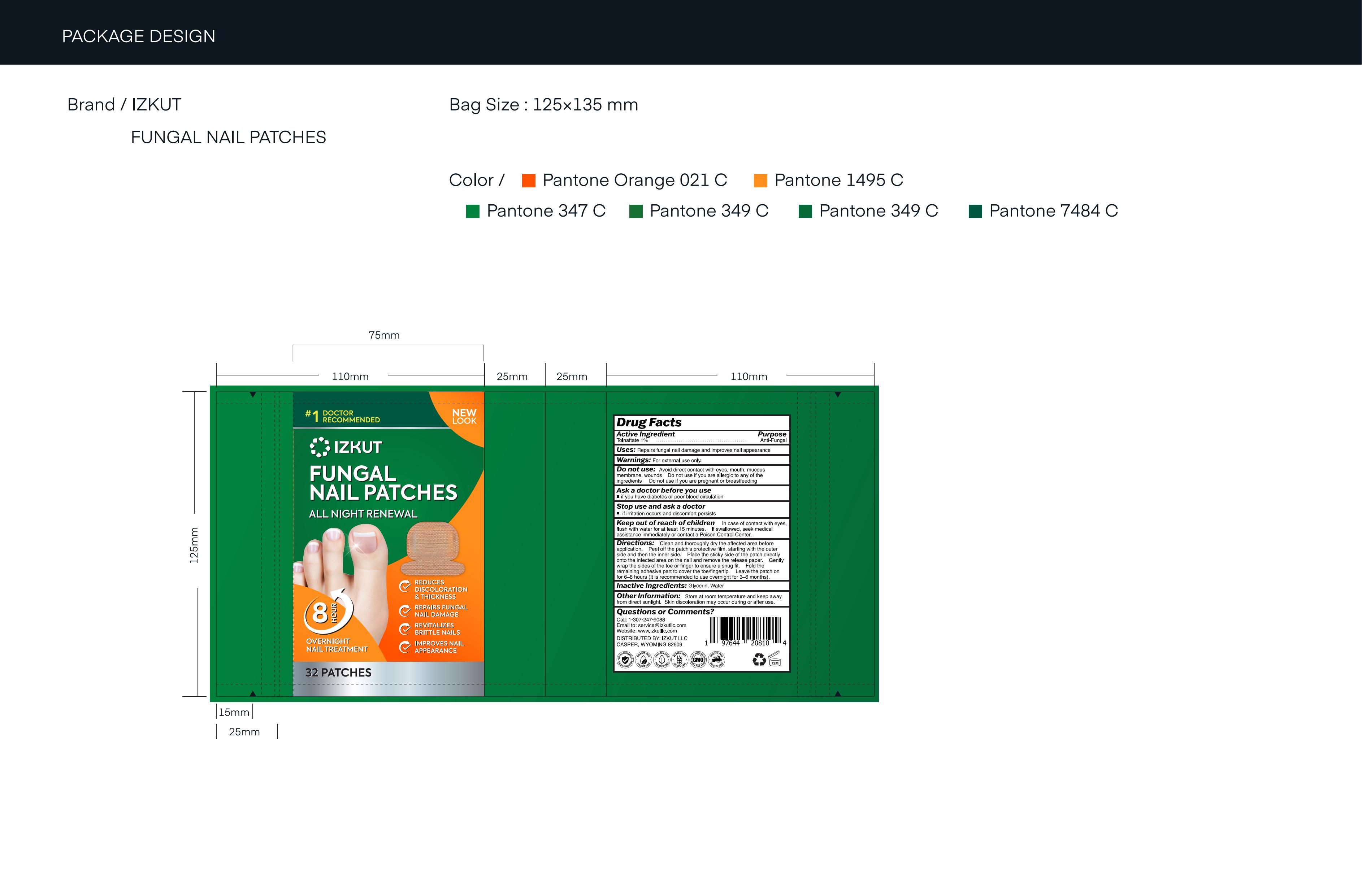
IZKUT FUNGAL NAIL PATCHES- fungal nail patches patch
Shenzhen Situya Trading Co., Ltd.
----------
82739-022 Complete
Do not use
■ Do not use if you are allergic to any of the ingredients
■ Do not use if you are pregnant or breastfeeding
Keep Oot Of Reach Of Children
■ In case of contact with eyes, flush with water for at least 15 minutes.
■ If swallowed, seek medical assistance immediately or contact a Poison Control Center.
Directions
■ Clean and thoroughly dry the affected area before application.
■ Peel off the patch's protective film, starting with the outer side and then the inner side.
■ Place the sticky side of the patch directly onto the infected area on the nail and remove the release paper.
■ Gently wrap the sides of the toe or finger to ensure a snug fit.
■ Fold the remaining adhesive part to cover the toe/fingertip.
■ Leave the patch on for 6–8 hours (It is recommended to use overnight for 3–6 months).
Other information
■ Store at room temperature and keep away from direct sunlight.
■ Skin discoloration may occur during or after use.
| IZKUT FUNGAL NAIL PATCHES
fungal nail patches patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Situya Trading Co., Ltd. | 706154255 | manufacture(82739-022) , label(82739-022) | |