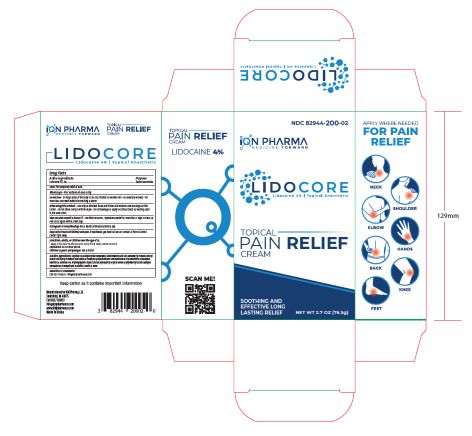
LIDOCORE- lidocaine cream
Lidocore by
Drug Labeling and Warnings
Lidocore by is a Otc medication manufactured, distributed, or labeled by iON Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- drug facts Active ingredients
- Uses
- Do not use section
- when using this product section
- Stop use section
- If pregnant or breastfeeding section
- Keep out of reach of children and pets section
- Directions section
-
INACTIVE INGREDIENT
Inactive Ingredients: Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe barbadensis Leaf Juice, Aminomethyl Propanol, Cetearyl
Alcohol (and) Dicetyl Phosphate (and) Ceteth-10 Phosphate, Cyclopentasiloxane (and) Dimethicone/Vinyl Dimethicone Crosspolymer,
Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glyceryl stearate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Phenoxyethanol, SD Alcohol, Steareth-21, Water. - Questions or comments section
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIDOCORE
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82944-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 3.1 g in 76.5 g Inactive Ingredients Ingredient Name Strength GLYCERYL 1-STEARATE (UNII: 258491E1RZ) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) STEARETH-21 (UNII: 53J3F32P58) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) DIMETHICONE 350 (UNII: 2Y53S6ATLU) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) ALCOHOL 95% (UNII: 7528N5H79B) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) WATER (UNII: 059QF0KO0R) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CETETH-10 PHOSPHATE (UNII: 4E05O5N49G) ISOHEXADECANE (UNII: 918X1OUF1E) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82944-201-02 1 in 1 CARTON 09/13/2024 1 76.5 g in 1 BOTTLE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/03/2024 Labeler - iON Pharma, LLC (118739596)
Trademark Results [Lidocore]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIDOCORE 98863806 not registered Live/Pending |
ION PHARMA L.L.C. 2024-11-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.