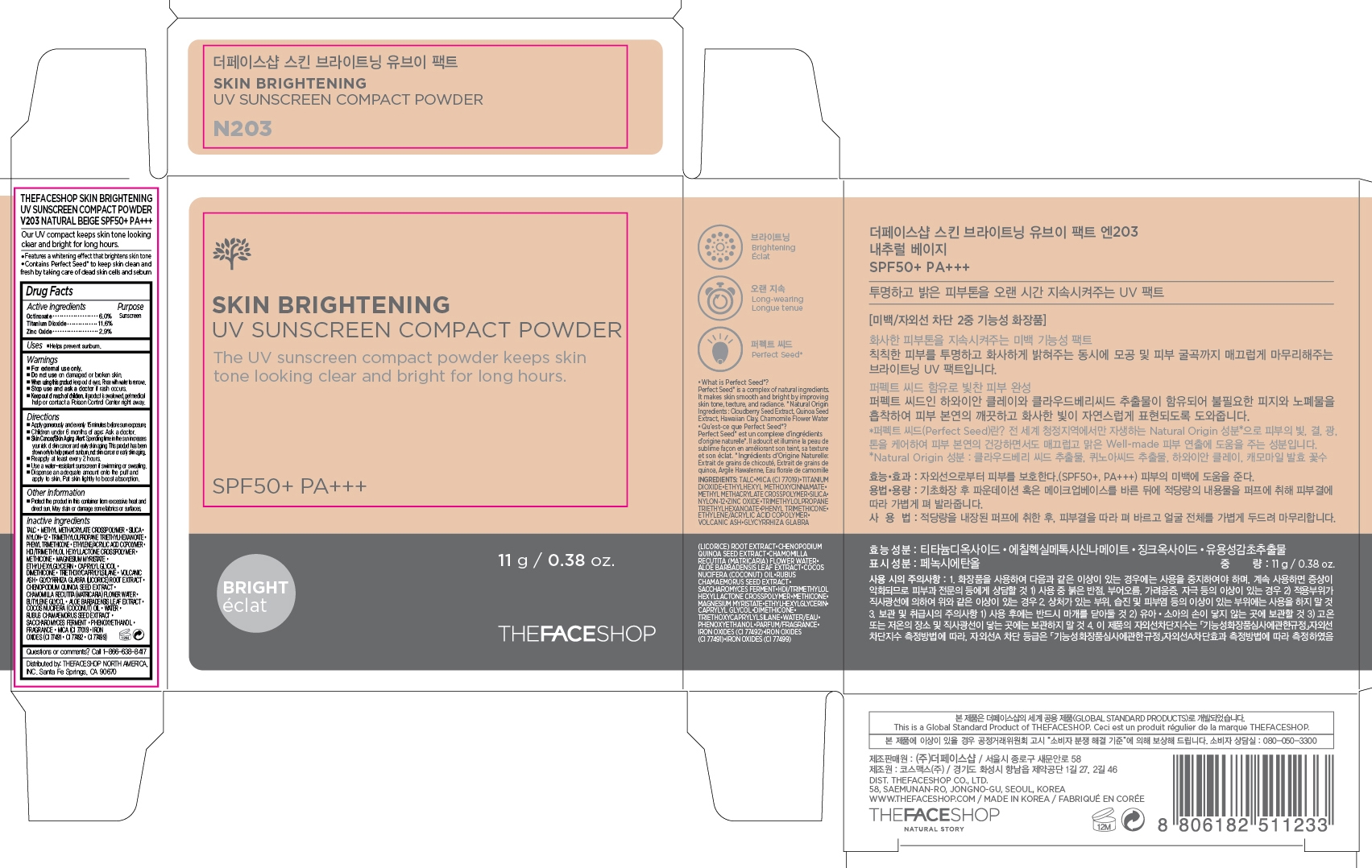
SKIN BRIGHTENING SPF 50 PA- octinoxate, titanium dioxide and zinc oxide powder powder
SKIN BRIGHTENING by
Drug Labeling and Warnings
SKIN BRIGHTENING by is a Otc medication manufactured, distributed, or labeled by THEFACESHOP CO., LTD., THEFACESHOP NORTH AMERICA, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
Apply generously and evenly 15 minutes before sun exposure.
Children under 6 months of age: Ask a doctor.
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Reapply at least every 2 hours.
Use a water-resistant sunscreen if swimming or sweating.Dispense an adequate amount onto the puff and apply to skin. Pat skin lightly to boost absorption.
- OTHER SAFETY INFORMATION
-
Inactive Ingredients
Talc, Methyl Methacrylate Crosspolymer, Silica, Nylon-12, Trimethylolpropane Triethylhexanoate, Phenyl Trimethicone, Ethylene/Acrylic Acid Copolymer, HDI/Trimethylol Hexyllactone Crosspolymer, Methicone, Magnesium Myristate, Ethylhexylglycerin, Caprylyl Glycol, Dimethicone, Triethoxycaprylylsilane, Volcanic Ash, Glycyrrhiza Glabra (Licorice) Root Extract, Chenopodium Quinoa Seed Extract, Chamomilla Recutita (Matricaria) Flower Water, Butylene Glycol, Aloe Barbadensis Leaf Extract, Cocos Nucifera (Coconut) Oil, Water, Rubus Chamaemorus Seed Extract, Saccharomyces Ferment, Phenoxyethanol, Fragrance, Mica (CI 77019), Iron Oxides (CI 77491, CI 77492, CI 77499)
- QUESTIONS
- Distributed by:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKIN BRIGHTENING SPF 50 PA
octinoxate, titanium dioxide and zinc oxide powder powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51523-373 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.66 g in 11 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.28 g in 11 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.32 g in 11 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51523-373-25 1 in 1 CARTON 10/01/2015 1 11 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/01/2015 Labeler - THEFACESHOP CO., LTD. (688329416) Registrant - THEFACESHOP NORTH AMERICA, INC. (620459193) Establishment Name Address ID/FEI Business Operations THEFACESHOP CO., LTD. 688329416 label(51523-373)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.