POLIVY- polatuzumab vedotin injection, powder, lyophilized, for solution
POLIVY by
Drug Labeling and Warnings
POLIVY by is a Prescription medication manufactured, distributed, or labeled by Genentech, Inc., Roche Singapore Technical Operations Pte. Ltd., Roche Diagnostics GmbH, F. Hoffmann-La Roche Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POLIVY safely and effectively. See full prescribing information for POLIVY.
POLIVY™ (polatuzumab vedotin-piiq) for injection, for intravenous use
Initial U.S. Approval: 2019INDICATIONS AND USAGE
POLIVY is a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma, not otherwise specified, after at least two prior therapies. (1)
Accelerated approval was granted for this indication based on complete response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
DOSAGE AND ADMINISTRATION
- The recommended dose of POLIVY is 1.8 mg/kg as an intravenous infusion over 90 minutes every 21 days for 6 cycles in combination with bendamustine and a rituximab product. Subsequent infusions may be administered over 30 minutes if the previous infusion is tolerated. (2)
- Premedicate with an antihistamine and antipyretic before POLIVY. (2)
- See Full Prescribing Information for instructions on preparation and administration. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 140 mg of polatuzumab vedotin-piiq as a lyophilized powder in a single-dose vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Peripheral Neuropathy: Monitor patients for peripheral neuropathy and modify or discontinue dose accordingly. (5.1)
- Infusion-Related Reactions: Premedicate with an antihistamine and antipyretic. Monitor patients closely during infusions. Interrupt or discontinue infusion for reactions. (5.2)
- Myelosuppression: Monitor complete blood counts. Manage using dose delays or reductions and growth factor support. Monitor for signs of infection. (5.3)
- Serious and Opportunistic Infections: Closely monitor patients for signs of bacterial, fungal, or viral infections. (5.4)
- Progressive Multifocal Leukoencephalopathy (PML): Monitor patients for new or worsening neurological, cognitive, or behavioral changes suggestive of PML. (5.5)
- Tumor Lysis Syndrome: Closely monitor patients with high tumor burden or rapidly proliferative tumors. (5.6)
- Hepatotoxicity: Monitor liver enzymes and bilirubin. (5.7)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception during treatment and for 3 months after the last dose. (5.8)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) included neutropenia, thrombocytopenia, anemia, peripheral neuropathy, fatigue, diarrhea, pyrexia, decreased appetite, and pneumonia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Concomitant use of strong CYP3A inhibitors or inducers has the potential to affect the exposure to unconjugated monomethyl auristatin E (MMAE). (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Management of Adverse Reactions
2.3 Recommended Prophylactic Medications
2.4 Instructions for Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Peripheral Neuropathy
5.2 Infusion-Related Reactions
5.3 Myelosuppression
5.4 Serious and Opportunistic Infections
5.5 Progressive Multifocal Leukoencephalopathy (PML)
5.6 Tumor Lysis Syndrome
5.7 Hepatotoxicity
5.8 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on POLIVY
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Diffuse Large B-cell Lymphoma
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
POLIVY in combination with bendamustine and a rituximab product is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, after at least two prior therapies.
Accelerated approval was granted for this indication based on complete response rate [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of POLIVY is 1.8 mg/kg administered as an intravenous infusion every 21 days for 6 cycles in combination with bendamustine and a rituximab product. Administer POLIVY, bendamustine, and a rituximab product in any order on Day 1 of each cycle. The recommended dose of bendamustine is 90 mg/m2/day on Days 1 and 2 when administered with POLIVY and a rituximab product. The recommended dose of rituximab product is 375 mg/m2 intravenously on Day 1 of each cycle.
If not already premedicated, administer an antihistamine and antipyretic at least 30 minutes prior to POLIVY. Administer the initial dose of POLIVY over 90 minutes. Monitor patients for infusion-related reactions during the infusion and for a minimum of 90 minutes following completion of the initial dose. If the previous infusion was well tolerated, the subsequent dose of POLIVY may be administered as a 30-minute infusion and patients should be monitored during the infusion and for at least 30 minutes after completion of the infusion.
If a planned dose of POLIVY is missed, administer as soon as possible. Adjust the schedule of administration to maintain a 21-day interval between doses.
2.2 Management of Adverse Reactions
Table 1 provides management guidelines for peripheral neuropathy, infusion-related reaction, and myelosuppression.
Table 1 Management of Peripheral Neuropathy, Infusion-Related Reaction, and Myelosuppression Event Dose Modification - * Severity on Day 1 of any cycle.
- † If primary cause is due to lymphoma, dose delay or reduction may not be needed.
Grade 2–3
Peripheral NeuropathyHold POLIVY dosing until improvement to Grade 1 or lower.
If recovered to Grade 1 or lower on or before Day 14, restart POLIVY with the next cycle at a permanently reduced dose of 1.4 mg/kg.
If a prior dose reduction to 1.4 mg/kg has occurred, discontinue POLIVY.
If not recovered to Grade 1 or lower on or before Day 14, discontinue POLIVY.Grade 4
Peripheral NeuropathyDiscontinue POLIVY. Grade 1–3
Infusion-Related ReactionInterrupt POLIVY infusion and give supportive treatment.
For the first instance of Grade 3 wheezing, bronchospasm, or generalized urticaria, permanently discontinue POLIVY.
For recurrent Grade 2 wheezing or urticaria, or for recurrence of any Grade 3 symptoms, permanently discontinue POLIVY.
Otherwise, upon complete resolution of symptoms, infusion may be resumed at 50% of the rate achieved prior to interruption. In the absence of infusion related symptoms, the rate of infusion may be escalated in increments of 50 mg/hour every 30 minutes.
For the next cycle, infuse POLIVY over 90 minutes. If no infusion-related reaction occurs, subsequent infusions may be administered over 30 minutes. Administer premedication for all cycles.Grade 4
Infusion-Related ReactionStop POLIVY infusion immediately.
Give supportive treatment.
Permanently discontinue POLIVY.Grade 3–4 Neutropenia*,† Hold all treatment until ANC recovers to greater than 1000/microliter.
If ANC recovers to greater than 1000/microliter on or before Day 7, resume all treatment without any additional dose reductions. Consider granulocyte colony-stimulating factor prophylaxis for subsequent cycles, if not previously given.
If ANC recovers to greater than 1000/microliter after Day 7:- restart all treatment. Consider granulocyte colony-stimulating factor prophylaxis for subsequent cycles, if not previously given. If prophylaxis was given, consider dose reduction of bendamustine.
- if dose reduction of bendamustine has already occurred, consider dose reduction of POLIVY to 1.4 mg/kg.
Grade 3–4 Thrombocytopenia*,† Hold all treatment until platelets recover to greater than 75,000/microliter.
If platelets recover to greater than 75,000/microliter on or before Day 7, resume all treatment without any additional dose reductions.
If platelets recover to greater than 75,000/microliter after Day 7:- restart all treatment, with dose reduction of bendamustine.
- if dose reduction of bendamustine has already occurred, consider dose reduction of POLIVY to 1.4 mg/kg.
2.3 Recommended Prophylactic Medications
If not already premedicated for a rituximab product, administer an antihistamine and antipyretic at least 30 to 60 minutes prior to POLIVY for potential infusion-related reactions [see Warnings and Precautions (5.2)].
Administer prophylaxis for Pneumocystis jiroveci pneumonia and herpesvirus throughout treatment with POLIVY.
Consider prophylactic granulocyte colony stimulating factor administration for neutropenia [see Warnings and Precautions (5.3)].
Administer tumor lysis syndrome prophylaxis for patients at increased risk of tumor lysis syndrome [see Warnings and Precautions (5.6)].
2.4 Instructions for Preparation and Administration
Reconstitute and further dilute POLIVY prior to intravenous infusion.
POLIVY is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Reconstitution
- Reconstitute immediately before dilution.
- More than one vial may be needed for a full dose. Calculate the dose, the total volume of reconstituted POLIVY solution required, and the number of POLIVY vials needed.
- Reconstitute each 140 mg POLIVY vial by using a sterile syringe to slowly inject 7.2 mL of Sterile Water for Injection, USP, with the stream directed toward the inside wall of the vial to obtain a concentration of 20 mg/mL of polatuzumab vedotin-piiq.
- Swirl the vial gently until completely dissolved. Do not shake.
- Inspect the reconstituted solution for discoloration and particulate matter. The reconstituted solution should appear colorless to slightly brown, clear to slightly opalescent, and free of visible particulates. Do not use if the reconstituted solution is discolored, is cloudy, or contains visible particulates. Do not freeze or expose to direct sunlight.
- If needed, store unused reconstituted POLIVY solution refrigerated at 2°C to 8°C (36°F to 46°F) for up to 48 hours or at room temperature (9°C to 25°C, 47°F to 77°F) up to a maximum of 8 hours prior to dilution. Discard vial when cumulative storage time prior to dilution exceeds 48 hours.
Dilution
- Dilute polatuzumab vedotin-piiq to a final concentration of 0.72–2.7 mg/mL in an intravenous infusion bag with a minimum volume of 50 mL containing 0.9% Sodium Chloride Injection, USP, 0.45% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP.
- Determine the volume of 20 mg/mL reconstituted solution needed based on the required dose.
- Withdraw the required volume of reconstituted solution from the POLIVY vial using a sterile syringe and dilute into the intravenous infusion bag. Discard any unused portion left in the vial.
- Gently mix the intravenous bag by slowly inverting the bag. Do not shake.
- Inspect the intravenous bag for particulates and discard if present.
- If not used immediately, store the diluted POLIVY solution as specified in Table 2. Discard if storage time exceeds these limits. Do not freeze or expose to direct sunlight.
Table 2 Diluted POLIVY Solution Storage Conditions Diluent Used to Prepare Solution for Infusion Diluted POLIVY Solution Storage Conditions* - * To ensure product stability, do not exceed specified storage durations.
0.9% Sodium Chloride Injection, USP Up to 24 hours at 2°C to 8°C (36°F to 46°F) or up to 4 hours at room temperature (9 to 25°C, 47 to 77°F) 0.45% Sodium Chloride Injection, USP Up to 18 hours at 2°C to 8°C (36°F to 46°F) or up to 4 hours at room temperature (9 to 25°C, 47 to 77°F) 5% Dextrose Injection, USP Up to 36 hours at 2°C to 8°C (36°F to 46°F) or up to 6 hours at room temperature (9 to 25°C, 47 to 77°F) - Limit transportation to 30 minutes at 9°C to 25°C or 12 hours at 2°C to 8°C (refer to instructions below). The total storage plus transportation times of the diluted product should not exceed the storage duration specified in Table 2.
- Agitation stress can result in aggregation. Limit agitation of diluted product during preparation and transportation to administration site. Do not transport diluted product through an automated system (e.g., pneumatic tube or automated cart). If the prepared solution will be transported to a separate facility, remove air from the infusion bag to prevent aggregation. If air is removed, an infusion set with a vented spike is required to ensure accurate dosing during the infusion.
- No incompatibilities have been observed between POLIVY and intravenous infusion bags with product-contacting materials of polyvinyl chloride (PVC) or polyolefins (PO) such as polyethylene (PE) and polypropylene (PP). No incompatibilities have been observed with infusion sets or infusion aids with product-contacting materials of PVC, PE, polyurethane (PU), polybutadiene (PBD), acrylonitrile butadiene styrene (ABS), polycarbonate (PC), polyetherurethane (PEU), fluorinated ethylene propylene (FEP), or polytetrafluorethylene (PTFE), or with filter membranes composed of polyether sulfone (PES) or polysulfone (PSU).
Administration
- Administer POLIVY as an intravenous infusion only.
- POLIVY must be administered using a dedicated infusion line equipped with a sterile, non-pyrogenic, low-protein-binding in-line or add-on filter (0.2- or 0.22-micron pore size) and a catheter.
- Do not mix POLIVY with or administer as an infusion with other drugs.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Peripheral Neuropathy
POLIVY can cause peripheral neuropathy, including severe cases. Peripheral neuropathy occurs as early as the first cycle of treatment and is a cumulative effect [see Adverse Reactions (6.1)]. POLIVY may exacerbate pre-existing peripheral neuropathy.
In Study GO29365, of 173 patients treated with POLIVY, 40% reported new or worsening peripheral neuropathy, with a median time to onset of 2.1 months. The peripheral neuropathy was Grade 1 in 26% of cases, Grade 2 in 12%, and Grade 3 in 2.3%. Peripheral neuropathy resulted in POLIVY dose reduction in 2.9% of treated patients, dose delay in 1.2%, and permanent discontinuation in 2.9%. Sixty-five percent of patients reported improvement or resolution of peripheral neuropathy after a median of 1 month, and 48% reported complete resolution.
The peripheral neuropathy is predominantly sensory; however, motor and sensorimotor peripheral neuropathy also occur. Monitor for symptoms of peripheral neuropathy such as hypoesthesia, hyperesthesia, paresthesia, dysesthesia, neuropathic pain, burning sensation, weakness, or gait disturbance. Patients experiencing new or worsening peripheral neuropathy may require a delay, dose reduction, or discontinuation of POLIVY [see Dosage and Administration (2.2)].
5.2 Infusion-Related Reactions
POLIVY can cause infusion-related reactions, including severe cases. Delayed infusion-related reactions as late as 24 hours after receiving POLIVY have occurred. With premedication, 7% of patients (12/173) in Study GO29365 reported infusion-related reactions after the administration of POLIVY. The reactions were Grade 1 in 67%, Grade 2 in 25%, and Grade 3 in 8%. Symptoms included fever, chills, flushing, dyspnea, hypotension, and urticaria.
Administer an antihistamine and antipyretic prior to the administration of POLIVY, and monitor patients closely throughout the infusion. If an infusion-related reaction occurs, interrupt the infusion and institute appropriate medical management [see Dosage and Administration (2.2)].
5.3 Myelosuppression
Treatment with POLIVY can cause serious or severe myelosuppression, including neutropenia, thrombocytopenia, and anemia. In patients treated with POLIVY plus BR (n = 45), 42% received primary prophylaxis with granulocyte colony-stimulating factor. Grade 3 or higher hematologic adverse reactions included neutropenia (42%), thrombocytopenia (40%), anemia (24%), lymphopenia (13%), and febrile neutropenia (11%) [see Adverse Reactions (6.1)]. Grade 4 hematologic adverse reactions included neutropenia (24%), thrombocytopenia (16%), lymphopenia (9%), and febrile neutropenia (4.4%). Cytopenias were the most common reason for treatment discontinuation (18% of all patients).
Monitor complete blood counts throughout treatment. Cytopenias may require a delay, dose reduction, or discontinuation of POLIVY [see Dosage and Administration (2.2)]. Consider prophylactic granulocyte colony-stimulating factor administration.
5.4 Serious and Opportunistic Infections
Fatal and/or serious infections, including opportunistic infections such as sepsis, pneumonia (including Pneumocystis jiroveci and other fungal pneumonia), herpesvirus infection, and cytomegalovirus infection have occurred in patients treated with POLIVY [see Adverse Reactions (6.1)].
Grade 3 or higher infections occurred in 32% (55/173) of patients treated with POLIVY. Infection-related deaths were reported in 2.9% of patients within 90 days of last treatment.
Closely monitor patients during treatment for signs of infection. Administer prophylaxis for Pneumocystis jiroveci pneumonia and herpesvirus.
5.5 Progressive Multifocal Leukoencephalopathy (PML)
PML has been reported after treatment with POLIVY (0.6%, 1/173). Monitor for new or worsening neurological, cognitive, or behavioral changes. Hold POLIVY and any concomitant chemotherapy if PML is suspected, and permanently discontinue if the diagnosis is confirmed.
5.6 Tumor Lysis Syndrome
POLIVY may cause tumor lysis syndrome. Patients with high tumor burden and rapidly proliferative tumor may be at increased risk of tumor lysis syndrome. Monitor closely and take appropriate measures, including tumor lysis syndrome prophylaxis.
5.7 Hepatotoxicity
Serious cases of hepatotoxicity that were consistent with hepatocellular injury, including elevations of transaminases and/or bilirubin, have occurred in patients treated with POLIVY.
In recipients of POLIVY in Study GO29365 (n = 173), Grade 3 and 4 transaminase elevations developed in 1.9% and 1.9%, respectively. Laboratory values suggestive of drug-induced liver injury (both an ALT or AST greater than 3 times upper limit of normal [ULN] and total bilirubin greater than 2 times ULN) occurred in 2.3% of patients.
Preexisting liver disease, elevated baseline liver enzymes, and concomitant medications may increase the risk of hepatotoxicity. Monitor liver enzymes and bilirubin level.
5.8 Embryo-Fetal Toxicity
Based on the mechanism of action and findings from animal studies, POLIVY can cause fetal harm when administered to a pregnant woman. The small molecule component of POLIVY, MMAE, administered to rats caused adverse developmental outcomes, including embryo-fetal mortality and structural abnormalities, at exposures below those occurring clinically at the recommended dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with POLIVY and for at least 3 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with POLIVY and for at least 5 months after the last dose [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the label:
- Peripheral Neuropathy [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Myelosuppression [see Warnings and Precautions (5.3)]
- Serious and Opportunistic Infections [see Warnings and Precautions (5.4)]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.5)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in this section reflect exposure to POLIVY in Study GO29365, a multicenter clinical trial for adult patients with relapsed or refractory B-cell lymphomas [see Clinical Studies (14)]. In patients with relapsed or refractory DLBCL, the trial included a single-arm safety evaluation of POLIVY in combination with bendamustine and a rituximab product (BR) (n = 6), followed by an open-label randomization to POLIVY in combination with BR versus BR alone (n = 39 treated per arm).
Following premedication with an antihistamine and antipyretic, POLIVY 1.8 mg/kg was administered by intravenous infusion on Day 2 of Cycle 1 and on Day 1 of Cycles 2–6, with a cycle length of 21 days. Bendamustine 90 mg/m2 daily was administered intravenously on Days 2 and 3 of Cycle 1 and on Days 1 and 2 of Cycles 2–6. A rituximab product dosed at 375 mg/m2 was administered intravenously on Day 1 of each cycle. Granulocyte colony-stimulating factor primary prophylaxis was optional and administered to 42% of recipients of POLIVY plus BR.
In POLIVY-treated patients (n = 45), the median age was 67 years (range 33 – 86) with 58% being ≥ age 65, 69% were male, 69% were white, and 87% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. The trial required an absolute neutrophil count ≥1500/µL, platelet count ≥75/µL, creatinine clearance (CLcr) ≥40 mL/min, hepatic transaminases ≤2.5 times ULN, and bilirubin <1.5 times ULN, unless abnormalities were from the underlying disease. Patients with Grade 2 or higher peripheral neuropathy or prior allogeneic hematopoietic stem cell transplantation (HSCT) were excluded.
Patients treated with POLIVY plus BR received a median of 5 cycles, with 49% receiving 6 cycles. Patients treated with BR alone received a median of 3 cycles, with 23% receiving 6 cycles.
Fatal adverse reactions occurred in 7% of recipients of POLIVY plus BR within 90 days of last treatment. Serious adverse reactions occurred in 64%, most often from infection. Serious adverse reactions in ≥5% of recipients of POLIVY plus BR included pneumonia (16%), febrile neutropenia (11%), pyrexia (9%), and sepsis (7%).
In recipients of POLIVY plus BR, adverse reactions led to dose reduction in 18%, dose interruption in 51%, and permanent discontinuation of all treatment in 31%. The most common adverse reactions leading to treatment discontinuation were thrombocytopenia and/or neutropenia.
Table 3 summarizes commonly reported adverse reactions. In recipients of POLIVY plus BR, adverse reactions in ≥20% of patients included neutropenia, thrombocytopenia, anemia, peripheral neuropathy, fatigue, diarrhea, pyrexia, decreased appetite, and pneumonia.
Table 3 Adverse Reactions Occurring in >10% of Patients with Relapsed or Refractory DLBCL and ≥5% More in the POLIVY Plus Bendamustine and Rituximab Product Group Adverse Reactions by Body System POLIVY + BR
n = 45BR
n = 39All Grades,
%Grade 3 or Higher,
%All Grades,
%Grade 3 or Higher,
%The table includes a combination of grouped and ungrouped terms. Events were graded using NCI CTCAE version 4. - * Includes 2 events with fatal outcome.
- † Includes 1 event with fatal outcome.
Blood and Lymphatic System Disorders Neutropenia 49 42 44 36 Thrombocytopenia 49 40 33 26 Anemia 47 24 28 18 Lymphopenia 13 13 8 8 Nervous System Disorders Peripheral neuropathy 40 0 8 0 Dizziness 13 0 8 0 Gastrointestinal Disorders Diarrhea 38 4.4 28 5 Vomiting 18 2.2 13 0 General Disorders Infusion-related reaction 18 2.2 8 0 Pyrexia 33 2.2 23 0 Decreased appetite 27 2.2 21 0 Infections Pneumonia 22 16* 15 2.6† Upper respiratory tract infection 13 0 8 0 Investigations Weight decreased 16 2.2 8 2.6 Metabolism and Nutrition Disorders Hypokalemia 16 9 10 2.6 Hypoalbuminemia 13 2.2 8 0 Hypocalcemia 11 2.2 5 0 Other clinically relevant adverse reactions (<10% or with a <5% difference) in recipients of POLIVY plus BR included:
- Blood and lymphatic system disorders: pancytopenia (7%)
- Musculoskeletal disorders: arthralgia (7%)
- Investigations: hypophosphatemia (9%), transaminase elevation (7%), lipase increase (7%)
- Respiratory disorders: pneumonitis (4.4%)
Selected treatment-emergent laboratory abnormalities are summarized in Table 4. In recipients of POLIVY plus BR, >20% of patients developed Grade 3 or 4 neutropenia, leukopenia, or thrombocytopenia, and >10% developed Grade 4 neutropenia (13%) or Grade 4 thrombocytopenia (11%).
Table 4 Selected Laboratory Abnormalities Worsening from Baseline in Patients with Relapsed or Refractory DLBCL and ≥5% More in the POLIVY Plus Bendamustine and Rituximab Product Group Laboratory Parameter* POLIVY + BR
n = 45BR
n = 39All Grades,
(%)Grade 3–4,
(%)All Grades,
(%)Grade 3–4,
(%)- * Includes laboratory abnormalities that are new or worsening in grade or with worsening from baseline unknown.
Hematologic Lymphocyte count decreased 87 87 90 82 Neutrophil count decreased 78 61 56 33 Hemoglobin decreased 78 18 62 10 Platelet count decreased 76 31 64 26 Chemistry Creatinine increased 87 4.4 77 5 Calcium decreased 44 9 26 0 SGPT/ALT increased 38 0 8 2.6 SGOT/AST increased 36 0 26 2.6 Lipase increased 36 9 13 5 Phosphorus decreased 33 7 28 8 Amylase increased 24 0 18 2.6 Potassium decreased 24 11 28 5 Safety was also evaluated in 173 adult patients with relapsed or refractory lymphoma who received POLIVY, bendamustine, and either a rituximab product or obinutuzumab in Study GO29365, including the 45 patients with DLBCL described above. In the expanded safety population, the median age was 66 years (range 27 – 86), 57% were male, 91% had an ECOG performance status of 0-1, and 32% had a history of peripheral neuropathy at baseline.
Fatal adverse reactions occurred in 4.6% of recipients of POLIVY within 90 days of last treatment, with infection as a leading cause. Serious adverse reactions occurred in 60%, most often from infection.
Table 5 summarizes the most common adverse reactions in the expanded safety population. The overall safety profile was similar to that described above. Adverse reactions in ≥20% of patients were diarrhea, neutropenia, peripheral neuropathy, fatigue, thrombocytopenia, pyrexia, decreased appetite, anemia, and vomiting. Infection-related adverse reactions in >10% of patients included upper respiratory tract infection, febrile neutropenia, pneumonia, and herpesvirus infection.
Table 5 Most Common Adverse Reactions (≥20% Any Grade or ≥5% Grade 3 or Higher) in Recipients of POLIVY and Chemoimmunotherapy for Relapsed or Refractory Lymphoma Adverse Reaction by Body System POLIVY + Bendamustine + Rituximab Product or Obinutuzumab
n = 173All Grades,
%Grade 3 or Higher,
%The table includes a combination of grouped and ungrouped terms. - * Primary prophylaxis with granulocyte colony-stimulating factor was given to 46% of all patients.
- † Includes 5 events with fatal outcome.
- ‡ Includes 4 events with fatal outcome.
Blood and Lymphatic System Disorders Neutropenia 44 39 Thrombocytopenia 31 23 Anemia 28 14 Febrile neutropenia* 13 13 Leukopenia 13 8 Lymphopenia 12 12 Nervous System Disorders Peripheral neuropathy 40 2.3 Gastrointestinal Disorders Diarrhea 45 8 Vomiting 27 2.9 General Disorders Fatigue 40 5 Pyrexia 30 2.9 Decreased appetite 29 1.7 Infections Pneumonia 13 10† Sepsis 6 6‡ Metabolism and Nutrition Disorders Hypokalemia 18 6 Other clinically relevant adverse reactions (<20% any grade) included:
- General disorders: infusion-related reaction (7%)
- Infection: upper respiratory tract infection (16%), lower respiratory tract infection (10%), herpesvirus infection (12%), cytomegalovirus infection (1.2%)
- Respiratory: dyspnea (19%), pneumonitis (1.7%)
- Nervous system disorders: dizziness (10%)
- Investigations: weight decrease (10%), transaminase elevation (8%), lipase increase (3.5%)
- Musculoskeletal disorders: arthralgia (7%)
- Eye disorders: blurred vision (1.2%)
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to polatuzumab vedotin-piiq in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Across all arms of Study GO29365, 8/134 (6%) patients tested positive for antibodies against polatuzumab vedotin-piiq at one or more post-baseline time points. Across clinical trials, 14/536 (2.6%) evaluable POLIVY-treated patients tested positive for such antibodies at one or more post-baseline time points. Due to the limited number of patients with antibodies against polatuzumab vedotin-piiq, no conclusions can be drawn concerning a potential effect of immunogenicity on efficacy or safety.
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on POLIVY
Strong CYP3A Inhibitors
Concomitant use with a strong CYP3A4 inhibitor may increase unconjugated MMAE AUC [see Clinical Pharmacology (12.3)], which may increase POLIVY toxicities. Monitor patients for signs of toxicity.
Strong CYP3A Inducers
Concomitant use with a strong CYP3A4 inducer may decrease unconjugated MMAE AUC [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], POLIVY can cause fetal harm. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of the small molecule component of POLIVY, MMAE, to pregnant rats during organogenesis at exposures below the clinical exposure at the recommended dose of 1.8 mg/kg POLIVY every 21 days resulted in embryo-fetal mortality and structural abnormalities (see Data). Advise a pregnant woman of the potential risks to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
No embryo-fetal development studies in animals have been performed with polatuzumab vedotin-piiq. In an embryo-fetal developmental study in pregnant rats, administration of two intravenous doses of MMAE, the small molecule component of POLIVY, on gestational days 6 and 13 caused embryo-fetal mortality and structural abnormalities, including protruding tongue, malrotated limbs, gastroschisis, and agnathia compared to controls at a dose of 0.2 mg/kg (approximately 0.5-fold the human area under the curve [AUC] at the recommended dose).
8.2 Lactation
Risk Summary
There is no information regarding the presence of polatuzumab vedotin-piiq in human milk, the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with POLIVY and for at least 2 months after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating POLIVY [see Use in Specific Populations (8.1)].
Contraception
Females
POLIVY can cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with POLIVY and for 3 months after the final dose [see Nonclinical Toxicology (13.1)].
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with POLIVY and for at least 5 months after the final dose [see Nonclinical Toxicity (13.1)].
Infertility
Based on findings from animal studies, POLIVY may impair male fertility. The reversibility of this effect is unknown [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of POLIVY have not been established in pediatric patients.
8.5 Geriatric Use
Among 173 patients treated with POLIVY in Study GO29365, 95 (55%) were ≥65 years of age. Patients aged ≥65 had a numerically higher incidence of serious adverse reactions (64%) than patients aged <65 (53%). Clinical studies of POLIVY did not include sufficient numbers of patients aged ≥65 to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
Avoid the administration of POLIVY in patients with moderate or severe hepatic impairment (bilirubin greater than 1.5 × ULN). Patients with moderate or severe hepatic impairment are likely to have increased exposure to MMAE, which may increase the risk of adverse reactions. POLIVY has not been studied in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3) and Warnings and Precautions (5.7)].
No adjustment in the starting dose is required when administering POLIVY to patients with mild hepatic impairment (bilirubin greater than ULN to less than or equal to 1.5 × ULN or AST greater than ULN).
-
11 DESCRIPTION
Polatuzumab vedotin-piiq is a CD79b-directed antibody-drug conjugate (ADC) consisting of three components: 1) the humanized immunoglobulin G1 (IgG1) monoclonal antibody specific for human CD79b; 2) the small molecule anti-mitotic agent MMAE; and 3) a protease-cleavable linker maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (mc-vc-PAB) that covalently attaches MMAE to the polatuzumab antibody.
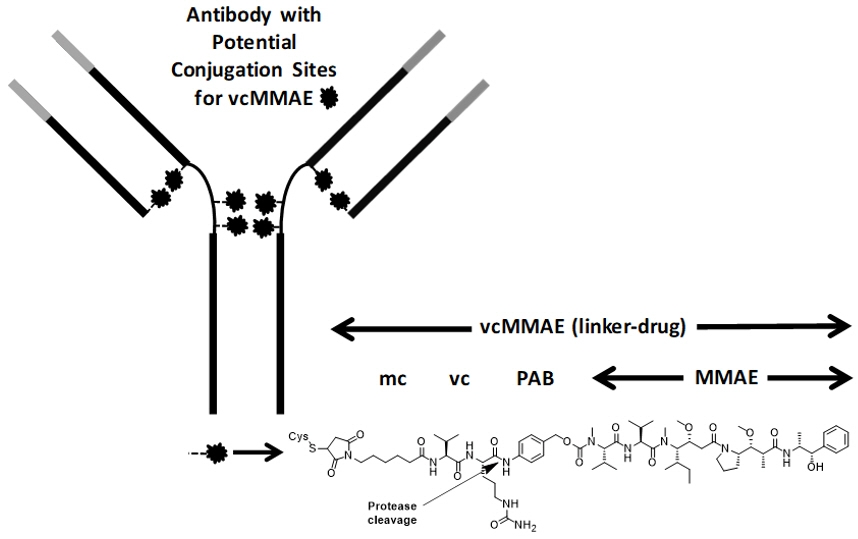
Polatuzumab vedotin-piiq has an approximate molecular weight of 150 kDa. An average of 3.5 molecules of MMAE are attached to each antibody molecule. Polatuzumab vedotin-piiq is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the small molecule components are produced by chemical synthesis.
POLIVY (polatuzumab vedotin-piiq) for injection is supplied as a sterile, white to grayish-white, preservative-free, lyophilized powder, which has a cake-like appearance, for intravenous infusion after reconstitution and dilution. After reconstitution with 7.2 mL of Sterile Water for Injection, USP, the final concentration is 20 mg/mL with a pH of approximately 5.3. Each single-dose vial delivers 140 mg of polatuzumab vedotin-piiq, polysorbate-20 (8.4 mg), sodium hydroxide (3.80 mg), succinic acid (8.27 mg), and sucrose (288 mg).
The POLIVY vial stoppers are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Polatuzumab vedotin-piiq is a CD79b-directed antibody-drug conjugate with activity against dividing B cells. The small molecule, MMAE, is an anti-mitotic agent covalently attached to the antibody via a cleavable linker. The monoclonal antibody binds to CD79b, a B-cell specific surface protein, which is a component of the B-cell receptor. Upon binding CD79b, polatuzumab vedotin-piiq is internalized, and the linker is cleaved by lysosomal proteases to enable intracellular delivery of MMAE. MMAE binds to microtubules and kills dividing cells by inhibiting cell division and inducing apoptosis.
12.2 Pharmacodynamics
Over polatuzumab vedotin-piiq dosages of 0.1 to 2.4 mg/kg (0.06 to 1.33 times the approved recommended dosage), a higher exposure was associated with higher incidence of some adverse reactions (e.g., ≥Grade 2 peripheral neuropathy, ≥Grade 3 anemia) and a lower exposure was associated with lower efficacy.
12.3 Pharmacokinetics
The exposure parameters of antibody-conjugated MMAE (acMMAE) and unconjugated MMAE (the cytotoxic component of polatuzumab vedotin-piiq) are summarized in Table 6. The plasma exposure of acMMAE and unconjugated MMAE increased proportionally over a polatuzumab vedotin-piiq dose range from 0.1 to 2.4 mg/kg (0.06 to 1.33 times the approved recommended dosage). Cycle 3 acMMAE AUC were predicted to increase by approximately 30% over Cycle 1 AUC, and achieved more than 90% of the Cycle 6 AUC. Unconjugated MMAE plasma exposures were <3% of acMMAE exposures, and the AUC and Cmax were predicted to decrease after repeated every-3-week dosing.
Table 6 Exposure Parameters of acMMAE and Unconjugated MMAE* acMMAE
Mean (± SD)Unconjugated MMAE
Mean (± SD)Cmax = maximum concentration, AUCinf = area under the concentration-time curve from time zero to infinity. - * After the first polatuzumab vedotin-piiq dose of 1.8 mg/kg.
Cmax (ng/mL) 803 (± 233) 6.82 (± 4.73) AUCinf (day*ng/mL) 1860 (± 966) 52.3 (± 18.0) Distribution
The acMMAE central volume of distribution estimated based on population PK analysis is 3.15 L. For humans, MMAE plasma protein binding is 71% to 77% and the blood-to-plasma ratio is 0.79 to 0.98, in vitro.
Elimination
The acMMAE terminal half-life is approximately 12 days (95% CI: 8.1 to 19.5 days) at Cycle 6 with predicted clearance of 0.9 L/day. The unconjugated MMAE terminal half-life is approximately 4 days after the first polatuzumab vedotin-piiq dose.
Specific Populations
No clinically significant differences in the pharmacokinetics of polatuzumab vedotin-piiq were observed based on age (20 to 89 years), sex, or race/ethnicity (Asian and non-Asian). No clinically significant differences in the pharmacokinetics of acMMAE or unconjugated MMAE were observed based on mild to moderate renal impairment (CLcr 30 to 89 mL/min). In mild hepatic impairment (AST or ALT >1.0 to 2.5 × ULN or total bilirubin >1.0 to 1.5 × ULN), there was a 40% increase in MMAE exposure, which was not deemed clinically significant.
The effect of severe renal impairment (CLcr 15 to 29 mL/min), end-stage renal disease with or without dialysis, moderate to severe hepatic impairment (AST or ALT >2.5 × ULN or total bilirubin >1.5 × ULN), or liver transplantation on the pharmacokinetics of acMMAE or unconjugated MMAE is unknown.
Drug Interaction Studies
No dedicated clinical drug-drug interaction studies with POLIVY in humans have been conducted.
Physiologically-Based Pharmacokinetic (PBPK) Modeling Predictions:
Strong CYP3A Inhibitor: Concomitant use of polatuzumab vedotin-piiq with ketoconazole (strong CYP3A inhibitor) is predicted to increase unconjugated MMAE AUC by 45%.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies in animals have not been performed with polatuzumab vedotin-piiq or MMAE.
MMAE was positive for genotoxicity in the in vivo rat bone marrow micronucleus study through an aneugenic mechanism. MMAE was not mutagenic in the bacterial reverse mutation (Ames) assay or the L5178Y mouse lymphoma forward mutation assay.
Fertility studies in animals have not been performed with polatuzumab vedotin-piiq or MMAE. However, results of repeat-dose toxicity in rats indicate the potential for polatuzumab vedotin-piiq to impair male fertility. In the 4-week repeat-dose toxicity study in rats with weekly dosing of 2, 6, and 10 mg/kg, dose-dependent testicular seminiferous tubule degeneration with abnormal lumen contents in the epididymis was observed. Findings in the testes and epididymis did not reverse and correlated with decreased testes weight and gross findings of small and/or soft testes at recovery necropsy in males given doses ≥2 mg/kg (below the exposure at the recommended dose based on unconjugated MMAE AUC).
-
14 CLINICAL STUDIES
14.1 Relapsed or Refractory Diffuse Large B-cell Lymphoma
The efficacy of POLIVY was evaluated in Study GO29365 (NCT02257567), an open-label, multicenter clinical trial that included a cohort of 80 patients with relapsed or refractory DLBCL after at least one prior regimen. Patients were randomized 1:1 to receive either POLIVY in combination with bendamustine and a rituximab product (BR) or BR alone for six 21-day cycles. Randomization was stratified by duration of response (DOR) to last therapy. Eligible patients were not candidates for autologous HSCT at study entry. The study excluded patients with Grade 2 or higher peripheral neuropathy, prior allogeneic HSCT, active central nervous system lymphoma, or transformed lymphoma.
Following premedication with an antihistamine and antipyretic, POLIVY was given by intravenous infusion at 1.8 mg/kg on Day 2 of Cycle 1 and on Day 1 of Cycles 2–6. Bendamustine was administered at 90 mg/m2 intravenously daily on Days 2 and 3 of Cycle 1 and on Days 1 and 2 of Cycles 2–6. A rituximab product was administered at a dose of 375 mg/m2 intravenously on Day 1 of Cycles 1–6. The cycle length was 21 days.
Of the 80 patients randomized to receive POLIVY plus BR (n = 40) or BR alone (n = 40), the median age was 69 years (range: 30–86 years), 66% were male, and 71% were white. Most patients (98%) had DLBCL not otherwise specified. The primary reasons patients were not candidates for HSCT included age (40%), insufficient response to salvage therapy (26%), and prior transplant failure (20%). The median number of prior therapies was 2 (range: 1–7), with 29% receiving one prior therapy, 25% receiving 2 prior therapies, and 46% receiving 3 or more prior therapies. Eighty percent of patients had refractory disease to last therapy.
In the POLIVY plus BR arm, patients received a median of 5 cycles, with 49% receiving 6 cycles. In the BR arm, patients received a median of 3 cycles, with 23% receiving 6 cycles.
Efficacy was based on complete response (CR) rate at the end of treatment and DOR, as determined by an independent review committee (IRC). Other efficacy measures included IRC-assessed best overall response.
Response rates are summarized in Table 7.
Table 7 Response Rates in Patients with Relapsed or Refractory DLBCL Response per IRC, n (%)* POLIVY + BR
n = 40BR
n = 40PR = partial remission. - * PET-CT based response per modified Lugano 2014 criteria. Bone marrow confirmation of PET-CT CR was required. PET-CT PR required meeting both PET criteria and CT criteria for PR.
- † End of treatment was defined as 6–8 weeks after Day 1 of Cycle 6 or last study treatment.
- ‡ Miettinen-Nurminen method.
- § PET-CT results were prioritized over CT results.
Objective Response at End of Treatment†
(95% CI)18 (45)
(29, 62)7 (18)
(7, 33)CR
(95% CI)16 (40)
(25, 57)7 (18)
(7, 33)Difference in CR rates, % (95% CI)‡ 22 (3, 41) Best Overall Response of CR or PR§
(95% CI)25 (63)
(46, 77)10 (25)
(13, 41)Best Response of CR
(95% CI)20 (50)
(34, 66)9 (23)
(11, 38)In the POLIVY plus BR arm, of the 25 patients who achieved a partial or complete response, 16 (64%) had a DOR of at least 6 months, and 12 (48%) had a DOR of at least 12 months. In the BR arm, of the 10 patients who achieved a partial or complete response, 3 (30%) had a DOR lasting at least 6 months, and 2 (20%) had a DOR lasting at least 12 months.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
POLIVY (polatuzumab vedotin-piiq) for injection is a preservative-free, white to grayish-white lyophilized powder, which has a cake-like appearance, supplied in a single-dose vial. Each carton (NDC: 50242-105-01) contains one 140 mg single-dose vial.
-
17 PATIENT COUNSELING INFORMATION
Peripheral Neuropathy
Advise patients that POLIVY can cause peripheral neuropathy. Advise patients to report to their healthcare provider any numbness or tingling of the hands or feet or any muscle weakness [see Warnings and Precautions (5.1)].
Infusion-Related Reactions
Advise patients to contact their healthcare provider if they experience signs and symptoms of infusion reactions, including fever, chills, rash, or breathing problems, within 24 hours of infusion [see Warnings and Precautions (5.2)].
Myelosuppression
Advise patients to report signs or symptoms of bleeding or infection immediately. Advise patients of the need for periodic monitoring of blood counts [see Warnings and Precautions (5.3)].
Infections
Advise patients to contact their healthcare provider if a fever of 38°C (100.4°F) or greater or other evidence of potential infection such as chills, cough, or pain on urination develops. Advise patients of the need for periodic monitoring of blood counts [see Warnings and Precautions (5.4)].
Progressive Multifocal Leukoencephalopathy
Advise patients to seek immediate medical attention for new or changes in neurological symptoms such as confusion, dizziness, or loss of balance; difficulty talking or walking; or changes in vision [see Warnings and Precautions (5.5)].
Tumor Lysis Syndrome
Advise patients to seek immediate medical attention for symptoms of tumor lysis syndrome such as nausea, vomiting, diarrhea, and lethargy [see Warnings and Precautions (5.6)].
Hepatotoxicity
Advise patients to report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with POLIVY [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1)].
Females and Males of Reproductive Potential
Advise females of reproductive potential, and males with female partners of reproductive potential, to use effective contraception during treatment with POLIVY and for at least 3 months and 5 months after the last dose, respectively [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed while receiving POLIVY and for at least 2 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
PRINCIPAL DISPLAY PANEL - 140 mg Vial Carton
NDC: 50242-105-01
Polivy™
(polatuzumab vedotin-piiq)
For Injection140 mg per vial
For Intravenous Infusion Only
Reconstitute and Dilute
prior to administration.
Single-Dose Vial.
Discard unused portion.CAUTION: Cytotoxic Agent
Rx only
1 vial
Genentech10215498

-
INGREDIENTS AND APPEARANCE
POLIVY
polatuzumab vedotin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50242-105 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Polatuzumab Vedotin (UNII: KG6VO684Z6) (Polatuzumab Vedotin - UNII:KG6VO684Z6) Polatuzumab Vedotin 140 mg in 7.52 mL Inactive Ingredients Ingredient Name Strength Succinic Acid (UNII: AB6MNQ6J6L) 8.27 mg in 7.52 mL Sodium Hydroxide (UNII: 55X04QC32I) 3.8 mg in 7.52 mL Sucrose (UNII: C151H8M554) 288 mg in 7.52 mL polysorbate 20 (UNII: 7T1F30V5YH) 8.4 mg in 7.52 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50242-105-01 1 in 1 CARTON 06/10/2019 1 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761121 06/10/2019 Labeler - Genentech, Inc. (080129000) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 080129000 API MANUFACTURE(50242-105) , ANALYSIS(50242-105) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 146373191 ANALYSIS(50242-105) Establishment Name Address ID/FEI Business Operations Roche Singapore Technical Operations Pte. Ltd. 937189173 ANALYSIS(50242-105) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 323105205 ANALYSIS(50242-105) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd 485244961 ANALYSIS(50242-105) , LABEL(50242-105) , PACK(50242-105) Establishment Name Address ID/FEI Business Operations Genentech, Inc. 833220176 LABEL(50242-105) , PACK(50242-105)
Trademark Results [POLIVY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 POLIVY 97415136 not registered Live/Pending |
Genentech, Inc. 2022-05-17 |
 POLIVY 87861879 5915678 Live/Registered |
Genentech, Inc. 2018-04-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.