NATACYN- natamycin suspension/ drops
NATACYN by
Drug Labeling and Warnings
NATACYN by is a Prescription medication manufactured, distributed, or labeled by Harrow Eye, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION:
NATACYN™ (natamycin ophthalmic suspension) 5% is a sterile, antifungal drug for topical ophthalmic administration. Each mL of NATACYN™ (natamycin ophthalmic suspension) contains: Active:natamycin 5% (50 mg). Preservative:benzalkonium chloride 0.02%. Inactive:sodium hydroxide and/or hydrochloric acid (neutralized to adjust the pH), purified water.
The active ingredient is represented by the chemical structure:
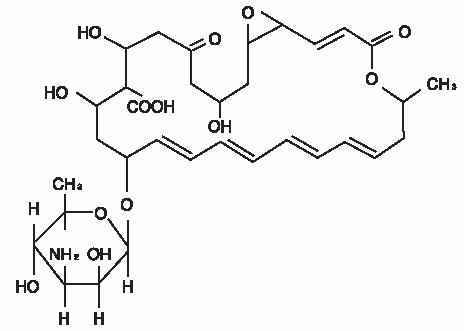
Established Name:Natamycin
Molecular Formula:C 33H 47NO 13
Molecular Weight:665.73 g/mol
Chemical Name:Stereoisomer of 22-[(3-amino-3,6-dideoxy- β-D-mannopyranosyl)oxy]-1,3,26- trihydroxy-12- methyl-10-oxo-6,11,28- trioxatricyclo[22.3.1.05,7] octacosa-8,14,16,18,20-pentaene-25- carboxylic acid.
Other:Pimaricin
The pH range is 5.0-7.5.
-
CLINICAL PHARMACOLOGY:
Natamycin is a tetraene polyene antibiotic derived from Streptomyces natalensis. It possesses in vitroactivity against a variety of yeast and filamentous fungi, including Candida, Aspergillus, Cephalosporium, Fusariumand Penicillium. The mechanism of action appears to be through binding of the molecule to the sterol moiety of the fungal cell membrane. The polyenesterol complex alters the permeability of the membrane to produce depletion of essential cellular constituents. Although the activity against fungi is dose-related, natamycin is predominantly fungicidal. Natamycin is not effective in vitroagainst gram-positive or gram-negative bacteria. Topical administration appears to produce effective concentrations of natamycin within the corneal stroma but not in intraocular fluid. Systemic absorption should not be expected following topical administration of NATACYN™ (natamycin ophthalmic suspension) 5%. As with other polyene antibiotics, absorption from the gastrointestinal tract is very poor. Studies in rabbits receiving topical natamycin revealed no measurable compound in the aqueous humor or sera, but the sensitivity of the measurement was no greater than 2 mg/mL.
-
INDICATIONS AND USAGE:
NATACYN™ (natamycin ophthalmic suspension) 5% is indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms including Fusarium solanikeratitis. As in other forms of suppurative keratitis, initial and sustained therapy of fungal keratitis should be determined by the clinical diagnosis, laboratory diagnosis by smear and culture of corneal scrapings and drug response. Whenever possible the in vitroactivity of natamycin against the responsible fungus should be determined. The effectiveness of natamycin as a single agent in fungal endophthalmitis has not been established.
- CONTRAINDICATIONS:
-
PRECAUTIONS:
General.
FOR TOPICAL OPHTHALMIC USE ONLY — NOT FOR INJECTION. Failure of improvement of keratitis following 7-10 days of administration of the drug suggests that the infection may be caused by a microorganism not susceptible to natamycin.
Continuation of therapy should be based on clinical re-evaluation and additional laboratory studies.
Adherence of the suspension to areas of epithelial ulceration or retention of the suspension in the fornices occurs regularly. Use only if the container is undamaged.
- Information for Patients:
- Carcinogenesis, Mutagenesis, Impairment of Fertility:
-
Pregnancy:
Animal reproduction studies have not been conducted with natamycin. It is also not known whether natamycin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. NATACYN ™(natamycin ophthalmic suspension) 5% should be given to a pregnant woman only if clearly needed.
- Nursing Mothers:
- Pediatric Use:
- Geriatric Use:
-
ADVERSE REACTIONS:
The following events have been identified during post-marketing use of NATACYN ™(natamycin ophthalmic suspension) 5% in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The events, which have been chosen for inclusion due to their seriousness, frequency of reporting, possible causal connection to NATACYN™ (natamycin ophthalmic suspension) 5%, or a combination of these factors include: allergic reaction, change in vision, chest pain, corneal opacity, dyspnea, eye discomfort, eye edema, eye hyperemia, eye irritation, eye pain, foreign body sensation, parethesia, and tearing.
-
DOSAGE AND ADMINISTRATION:
SHAKE WELL BEFORE USING. The preferred initial dosage in fungal keratitis is one drop of NATACYN™ (natamycin ophthalmic suspension) 5% instilled in the conjunctival sac at hourly or two-hourly intervals. The frequency of application can usually be reduced to one drop 6 to 8 times daily after the first 3 to 4 days. Therapy should generally be continued for 14 to 21 days or until there is resolution of active fungal keratitis. In many cases, it may be helpful to reduce the dosage gradually at 4 to 7 day intervals to assure that the replicating organism has been eliminated. Less frequent initial dosage (4 to 6 daily applications) may be sufficient in fungal blepharitis and conjunctivitis.
-
HOW SUPPLIED:
NATACYN™ (natamycin ophthalmic suspension) 5% is a 15 mL fill packaged in a 15 mL amber glass bottle with a black closure. A flint glass dropper with a red plastic closure and a black rubber bulb are packaged separately in a clear plastic blister with Tyvek backing.
15 mL NDC82667-012-05
STORAGE:Store between 2°C to 24°C (36°F-75°F). Do not freeze. Avoid exposure to light and excessive heat.
Rx Only
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
NDC: 82667-012-05
Harrow ®
Natacyn™
(natamycin ophthalmic suspension) 5%
Anti-Fungal Ophthalmic Suspension
Rx Only15 mL
Sterile

-
INGREDIENTS AND APPEARANCE
NATACYN
natamycin suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82667-012 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NATAMYCIN (UNII: 8O0C852CPO) (NATAMYCIN - UNII:8O0C852CPO) NATAMYCIN 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82667-012-05 1 in 1 CARTON 11/01/2024 1 15 mL in 1 BOTTLE, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050514 11/01/2024 Labeler - Harrow Eye, LLC (118526951)
Trademark Results [NATACYN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NATACYN 73243184 1182659 Live/Registered |
ALCON LABORATORIES, INC. 1979-12-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.