AMIKACIN SULFATE injection, solution
Amikacin Sulfate by
Drug Labeling and Warnings
Amikacin Sulfate by is a Prescription medication manufactured, distributed, or labeled by Qilu Pharmaceutical Co., Ltd., Qilu Pharmaceutical Co., Ltd. (Biological Industrial Park). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNINGS
Patients treated with parenteral aminoglycosides should be under close clinical observation because of the potential ototoxicity and nephrotoxicity associated with their use. Safety for treatment periods which are longer than 14 days has not been established.
Neurotoxicity, manifested as vestibular and permanent bilateral auditory ototoxicity, can occur in patients with preexisting renal damage and in patients with normal renal function treated at higher doses and/or for periods longer than those recommended. The risk of aminoglycoside-induced ototoxicity is greater in patients with renal damage. High frequency deafness usually occurs first and can be detected only by audiometric testing. Vertigo may occur and may be evidence of vestibular injury. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching and convulsions. The risk of hearing loss due to aminoglycosides increases with the degree of exposure to either high peak or high trough serum concentrations. Patients developing cochlear damage may not have symptoms during therapy to warn them of developing eighth-nerve toxicity, and total or partial irreversible bilateral deafness may occur after the drug has been discontinued. Aminoglycoside-induced ototoxicity is usually irreversible. Aminoglycosides are potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high doses or prolonged therapy.
Neuromuscular blockade and respiratory paralysis have been reported following parenteral injection, topical instillation (as in orthopedic and abdominal irrigation or in local treatment of empyema), and following oral use of aminoglycosides. The possibility of these phenomena should be considered if aminoglycosides are administered by any route, especially in patients receiving anesthetics, neuromuscular blocking agents such as tubocurarine, succinylcholine, decamethonium, or in patients receiving massive transfusions of citrate - anticoagulated blood. If blockage occurs, calcium salts may reverse these phenomena, but mechanical respiratory assistance may be necessary.
Renal and eighth-nerve function should be closely monitored especially in patients with known or suspected renal impairment at the onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Serum concentrations of amikacin should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels and prolonged peak concentrations above 35 micrograms per mL. Urine should be examined for decreased specific gravity, increased excretion of proteins, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be measured periodically. Serial audiograms should be obtained where feasible in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears, and hearing loss) or nephrotoxicity requires discontinuation of the drug or dosage adjustment.
Concurrent and/or sequential systemic, oral or topical use of other neurotoxic or nephrotoxic products, particularly bacitracin, cisplatin, amphotericin B, cephaloridine, paromomycin, viomycin, polymyxin B, colistin, vancomycin, or other aminoglycosides should be avoided. Other factors that may increase risk of toxicity are advanced age and dehydration.
The concurrent use of amikacin with potent diuretics (ethacrynic acid, or furosemide) should be avoided since diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering antibiotic concentrations in serum and tissue.
-
DESCRIPTION
Amikacin Sulfate Injection, USP is semi-synthetic aminoglycoside antibiotic derived from kanamycin. It is C22H43N5O132H2SO4O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[6-amino-6-deoxy-α-D-glucopyranosyl-(1→6)]-N3-(4-amino-L-2-hydroxybutyryl)-2-deoxy-L-streptamine sulfate (1:2)
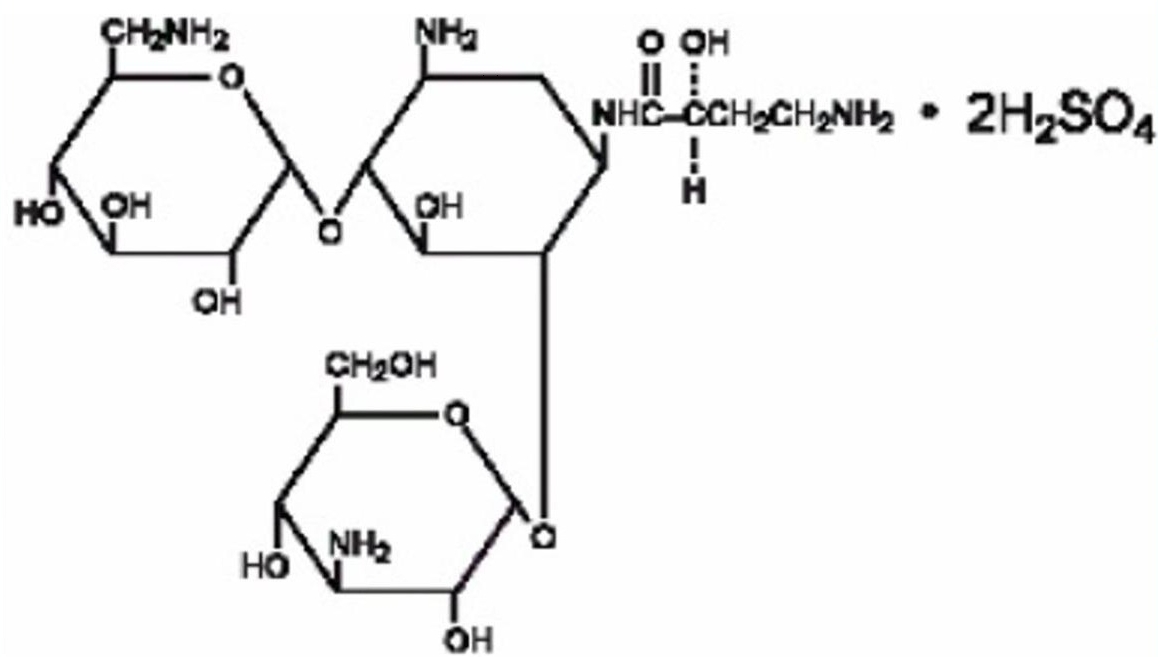
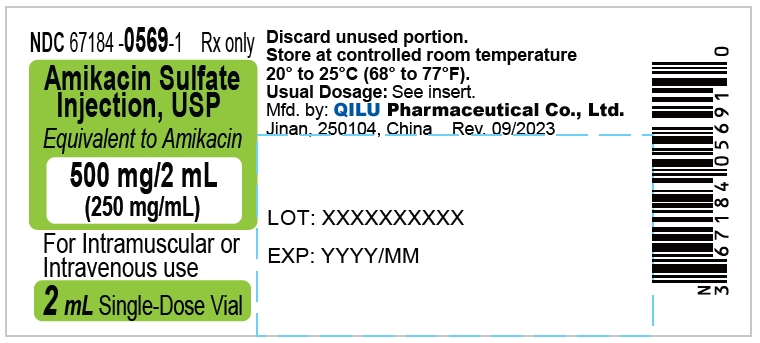
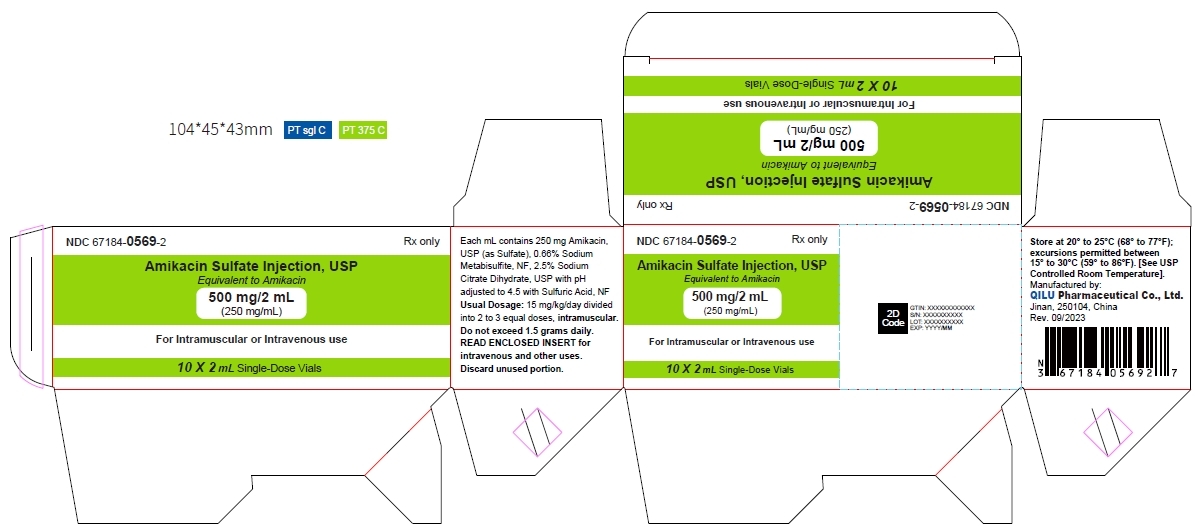
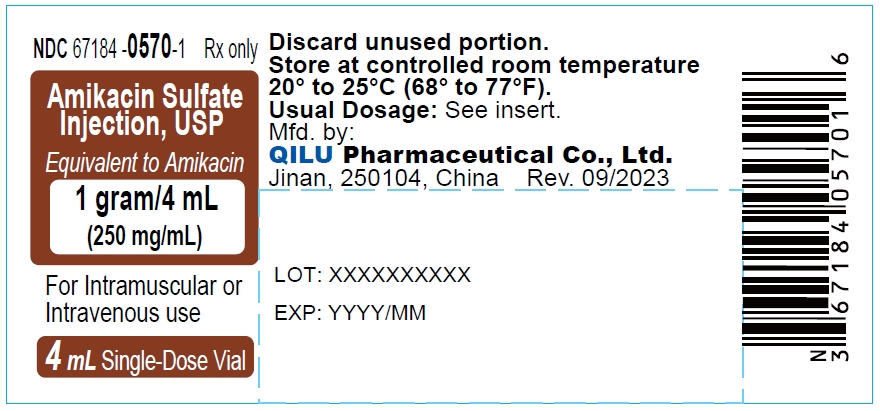
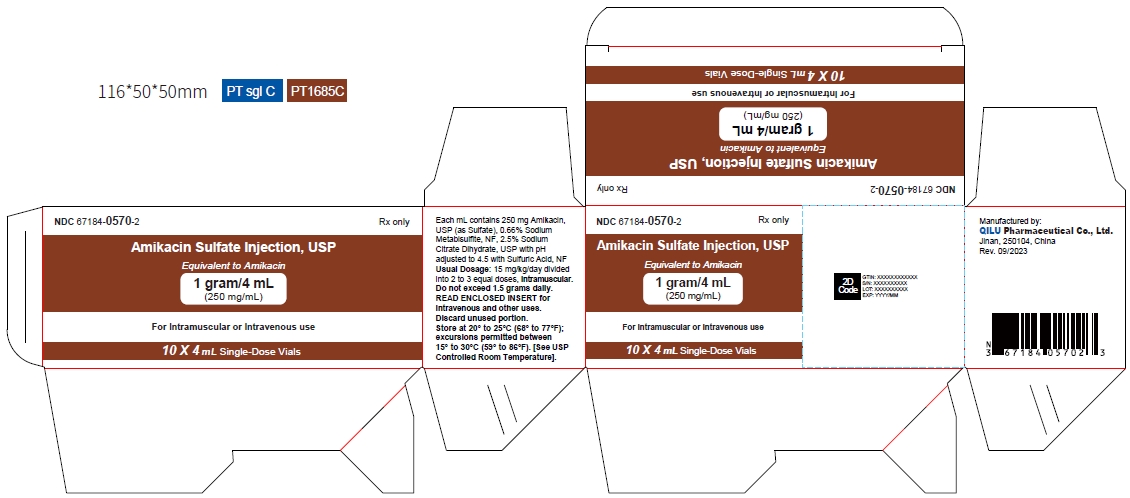
The dosage form is supplied as a sterile, colorless to light straw colored solution for intramuscular or intravenous use. The 500 mg per 2 mL vial and 1 gram per 4 mL vial contains per each mL: 250 mg Amikacin (as the Amikacin sulfate USP), 0.66% sodium metabisulfite as an antioxidant, 2.5% sodium citrate dihydrate as a buffering agent with pH adjusted to 4.5 with sulfuric acid.
-
CLINICAL PHARMACOLOGY
Amikacin is rapidly absorbed after intramuscular administration. In normal adult volunteers, average peak serum concentrations of about 12, 16, and 21 mcg/mL are obtained 1 hour after intramuscular administration of 250 mg (3.7 mg/kg), 375 mg (5 mg/kg), 500 mg (7.5 mg/kg), single doses, respectively. At 10 hours, serum levels are about 0.3 mcg/mL, 1.2 mcg/mL, and 2.1 mcg/mL, respectively.
Tolerance studies in normal volunteers reveal that amikacin is well tolerated locally following repeated intramuscular dosing, and when given at maximally recommended doses, no ototoxicity or nephrotoxicity has been reported. There is no evidence of drug accumulation with repeated dosing for 10 days when administered according to recommended doses.
With normal renal function, about 91.9% of an intramuscular dose is excreted unchanged in the urine in the first 8 hours, and 98.2% within 24 hours. Mean urine concentrations for 6 hours are 563 mcg/mL following a 250 mg dose, 697 mcg/mL following a 375 mg dose, and 832 mcg/mL following a 500 mg dose.
Preliminary intramuscular studies in newborns of different weights (less than 1.5 kg, 1.5 to 2 kg, over 2 kg) at a dose of 7.5 mg/kg revealed that, like other aminoglycosides, serum half-life values were correlated inversely with post-natal age and renal clearances of amikacin. The volume of distribution indicates that amikacin, like other aminoglycosides, remains primarily in the extracellular fluid space of neonates. Repeated dosing every 12 hours in all the above groups did not demonstrate accumulation after 5 days.
Intravenous Administration
Single doses of 500 mg (7.5 mg/kg) administered to normal adults as an infusion over a period of 30 minutes produced a mean peak serum concentration of 38 mcg/mL at the end of the infusion, and levels of 24 mcg/mL, 18 mcg/mL, and 0.75 mcg/mL at 30 minutes, 1 hour, and 10 hours post-infusion, respectively. Eighty-four percent of the administered dose was excreted in the urine in 9 hours and about 94% within 24 hours. Repeat infusions of 7.5 mg/kg every 12 hours in normal adults were well tolerated and caused no drug accumulation.
General
Pharmacokinetic studies in normal adult subjects reveal the mean serum half-life to be slightly over 2 hours with a mean total apparent volume of distribution of 24 liters (28% of the body weight). By the ultrafiltration technique, reports of serum protein binding range from 0 to 11%. The mean serum clearance rate is about 100 mL/min and the renal clearance rate is 94 mL/min in subjects with normal renal function.
Amikacin is excreted primarily by glomerular filtration. Patients with impaired renal function or diminished glomerular filtration pressure excrete the drug much more slowly (effectively prolonging the serum half-life). Therefore, renal function should be monitored carefully and dosage adjusted accordingly (see suggested dosage schedule under DOSAGE AND ADMINISTRATION)
Following administration at the recommended dose, therapeutic levels are found in bone, heart, gallbladder, and lung tissue in addition to significant concentrations in urine, bile, sputum, bronchial secretions, interstitial, pleural, and synovial fluids.
Spinal fluid levels in normal infants are approximately 10 to 20% of the serum concentrations and may reach 50% when the meninges are inflamed. Amikacin has been demonstrated to cross the placental barrier and yield significant concentrations in amniotic fluid. The peak fetal serum concentration is about 16% of the peak maternal serum concentration and maternal and fetal serum half-life values are about 2 and 3.7 hours, respectively.
Microbiology
Mechanism of Action
Amikacin, an aminoglycoside, binds to the prokaryotic ribosome, inhibiting protein synthesis in susceptible bacteria. It is bactericidal in vitro against Gram-positive and Gram-negative bacteria.
Mechanism of Resistance
Aminoglycosides are known to be ineffective against Salmonella and Shigella species in patients. Therefore, in vitro susceptibility test results should not be reported.
Amikacin resists degradation by certain aminoglycoside inactivating enzymes known to affect gentamicin, tobramycin, and kanamycin.
Aminoglycosides in general have a low order of activity against Gram-positive organisms other than Staphylococcal isolates.
Interaction with Other Antimicrobials
In vitro studies have shown that amikacin sulfate combined with a beta-lactam antibiotic acts synergistically against many clinically significant Gram-negative organisms.
Antimicrobial Activity
Amikacin has been shown to be active against the following bacteria, both in vitro and in clinical infections [see Indications and Usage]
Gram-positive Bacteria
Staphylococcus species
Gram-negative Bacteria
Pseudomonas species
Escherichia coli
Proteus species (indole-positive and indole-negative)
Klebsiella species
Enterobacter species
Serratia species
Acinetobacter species
Amikacin has demonstrated in vitro activity against the following bacteria. The safety and effectiveness of amikacin in treating clinical infections due to these bacteria have not been established in adequate and well-controlled trials.
Citrobacter freundii
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
INDICATIONS AND USAGE
Amikacin Sulfate Injection is indicated in the short-term treatment of serious infections due to susceptible strains of Gram-negative bacteria, including Pseudomonas species, Escherichia coli, species of indole-positive and indole-negative Proteus, Providencia species, Klebsiella-Enterobacter-Serratia species, and Acinetobacter (Mima-Herellea) species.
Clinical studies have shown Amikacin Sulfate Injection to be effective in bacterial septicemia (including neonatal sepsis); in serious infections of the respiratory tract, bones and joints, central nervous system (including meningitis) and skin and soft tissue; intra-abdominal infections (including peritonitis); and in burns and post-operative infections (including post-vascular surgery). Clinical studies have shown amikacin also to be effective in serious complicated and recurrent urinary tract infections due to these organisms. Aminoglycosides, including Amikacin Sulfate Injection are not indicated in uncomplicated initial episodes of urinary tract infections unless the causative organisms are not susceptible to antibiotics having less potential toxicity.
Bacteriologic studies should be performed to identify causative organisms and their susceptibilities to amikacin. Amikacin may be considered as initial therapy in suspected Gram-negative infections and therapy may be instituted before obtaining the results of susceptibility testing. Clinical trials demonstrated that amikacin was effective in infections caused by gentamicin and/or tobramycin-resistant strains of Gram-negative organisms, particularly Proteus rettgeri, Providencia stuartii, Serratia marcescens, and Pseudomonas aeruginosa. The decision to continue therapy with the drug should be based on results of the susceptibility tests, the severity of the infection, the response of the patient and the important additional considerations contained in the WARNINGS box above.
Amikacin has also been shown to be effective in staphylococcal infections and may be considered as initial therapy under certain conditions in the treatment of known or suspected staphylococcal disease such as, severe infections where the causative organism may be either a Gram-negative bacterium or a staphylococcus, infections due to susceptible strains of staphylococci in patients allergic to other antibiotics, and in mixed staphylococci/Gram-negative infections.
In certain severe infections such as neonatal sepsis, concomitant therapy with a penicillin-type drug may be indicated because of the possibility of infections due to Gram-positive organisms such as streptococci or pneumococci.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of amikacin and other antibacterial drugs, amikacin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy.
In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
- See WARNINGS box above.
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides cross the placenta and there have been several reports of total irreversible, bilateral congenital deafness in children whose mothers received streptomycin during pregnancy. Although serious side effects to the fetus or newborns have not been reported in the treatment of pregnant women with other aminoglycosides, the potential for harm exists. Reproduction studies of amikacin have been performed in rats and mice and revealed no evidence of impaired fertility or harm to the fetus due to amikacin. There are no well controlled studies in pregnant women, but investigational experience does not include any positive evidence of adverse effects to the fetus. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than nonasthmatic people.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Amikacin Sulfate Injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C.difficile.
C.difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C.difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C.difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C.difficile, and surgical evaluation should be instituted as clinically indicated.
Risk of Ototoxicity Due to Mitochondrial DNA Variants
Cases of ototoxicity with aminoglycosides have been observed in patients with certain variants in the mitochondrially encoded 12S rRNA gene (MT-RNR1), particularly the m.1555A>G variant. Ototoxicity occurred in some patients even when their aminoglycoside serum levels were within the recommended range. Mitochondrial DNA variants are present in less than 1% of the general US population, and the proportion of the variant carriers who may develop ototoxicity as well as the severity of ototoxicity is unknown. In case of known maternal history of ototoxicity due to aminoglycoside use or a known mitochondrial DNA variant in the patient, consider alternative treatments other than aminoglycosides unless the increased risk of permanent hearing loss is outweighed by the severity of infection and lack of safe and effective alternative therapies.
-
PRECAUTIONS
General
Prescribing amikacin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Aminoglycosides are quickly and almost totally absorbed when they are applied topically, except to the urinary bladder, in association with surgical procedures. Irreversible deafness, renal failure, and death due to neuromuscular blockade have been reported following irrigation of both small and large surgical fields with an aminoglycoside preparation.
Amikacin Sulfate Injection is potentially nephrotoxic, ototoxic and neurotoxic. The concurrent or serial use of other ototoxic or nephrotoxic agents should be avoided either systemically or topically because of the potential for additive effects. Increased nephrotoxicity has been reported following concomitant parenteral administration of aminoglycoside antibiotics and cephalosporins.
Concomitant cephalosporins may spuriously elevate creatinine determinations.
Since amikacin is present in high concentrations in the renal excretory system, patients should be well hydrated to minimize chemical irritation of the renal tubules. Kidney function should be assessed by the usual methods prior to starting therapy and daily during the course of treatment. If signs of renal irritation appear (casts, white or red cells, or albumin), hydration should be increased. A reduction in dosage (see DOSAGE AND ADMINISTRATION) may be desirable if other evidence of renal dysfunction occurs such as decreased creatinine clearance; decreased urine specific gravity; increased BUN, creatinine, or oliguria. If azotemia increases or if a progressive decrease in urinary output occurs, treatment should be stopped.
Note: When patients are well-hydrated and kidney function is normal the risk of nephrotoxic reactions with amikacin is low if the dosage recommendations (see DOSAGE AND ADMINISTRATION) are not exceeded.
Elderly patients may have reduced renal function which may not be evident in routine screening tests such as BUN or serum creatinine. A creatinine clearance determination may be more useful. Monitoring of renal function during treatment with aminoglycosides is particularly important. Aminoglycosides should be used with caution in patients with muscular disorders such as myasthenia gravis or parkinsonism since these drugs may aggravate muscle weakness because of their potential curare-like effect on the neuromuscular junction.
In vitro mixing of aminoglycosides with beta-lactam antibiotics (penicillin or cephalosporin) may result in a significant mutual inactivation. A reduction in serum half-life or serum level may occur when an aminoglycoside or penicillin-type drug is administered by separate routes. Inactivation of the aminoglycoside is clinically significant only in patients with severely impaired renal function. Inactivation may continue in specimens of body fluids collected for assay, resulting in inaccurate aminoglycoside readings. Such specimens should be properly handled (assayed promptly, frozen, or treated with beta-lactamase).
Cross-allergenicity among aminoglycosides has been demonstrated.
As with other antibiotics, the use of amikacin may result in overgrowth of nonsusceptible organisms. If this occurs, appropriate therapy should be instituted.
Aminoglycosides should not be given concurrently with potent diuretics (see WARNINGS box).
Information for Patients
Patients should be counseled that antibacterial drugs including amikacin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When amikacin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by amikacin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals to evaluate carcinogenic potential have not been performed, and mutagenicity has not been studied. Amikacin administered subcutaneously to rats at doses up to 4 times the human daily dose did not impair male or female fertility.
Nursing Mothers
It is not known whether amikacin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from amikacin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
All aminoglycosides have the potential to induce auditory, vestibular, and renal toxicity and neuromuscular blockade (see WARNINGS box). They occur more frequently in patients with present or past history of renal impairment, of treatment with other ototoxic or nephrotoxic drugs, and in patients treated for longer periods and/or with higher doses than recommended.
Neurotoxicity-Ototoxicity
Toxic effects on the eighth cranial nerve can result in hearing loss, loss of balance, or both. Amikacin primarily affects auditory function. Cochlear damage includes high frequency deafness and usually occurs before clinical hearing loss can be detected.
Neurotoxicity-Neuromuscular Blockade
Acute muscular paralysis and apnea can occur following treatment with aminoglycoside drugs.
Nephrotoxicity
Elevation of serum creatinine, albuminuria, presence of red and white cells, casts, azotemia, and oliguria have been reported. Renal function changes are usually reversible when the drug is discontinued. As would be expected with any aminoglycoside, reports of toxic nephropathy and acute renal failure have been received during postmarketing surveillance.
Other
In addition to those described above, other adverse reactions which have been reported on rare occasions are skin rash, drug fever, headache, paresthesia, tremor, nausea and vomiting, eosinophilia, arthralgia, anemia, hypotension and hypomagnesemia. Macular infarction sometimes leading to permanent loss of vision has been reported following intravitreous administration (injection into the eye) of amikacin.
To report SUSPECTED ADVERSE EVENTS, contact QILU Pharma, INC. at 484-838-0633 / 484-875-3013 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The patient's pretreatment body weight should be obtained for calculation of correct dosage. Amikacin Sulfate Injection may be given intramuscularly or intravenously.
The status of renal function should be estimated by measurement of the serum creatinine concentration or calculation of the endogenous creatinine clearance rate.
The blood urea nitrogen (BUN) is much less reliable for this purpose. Reassessment of renal function should be made periodically during therapy. Whenever possible, amikacin concentrations in serum should be measured to assure adequate but not excessive levels. It is desirable to measure both peak and trough serum concentrations intermittently during therapy. Peak concentrations (30 to 90 minutes after injection) above 35 micrograms per mL and trough concentrations (just prior to the next dose) above 10 micrograms per mL should be avoided. Dosage should be adjusted as indicated.
Since the vials are for single-dose only, any unused portion remaining in the vial should be discarded.
Intramuscular Administration for Patients with Normal Renal Function
The recommended dosage for adults, children and older infants (see WARNINGS box) with normal renal function is 15 mg/kg/day divided into 2 or 3 equal doses administered at equally-divided intervals, i.e., 7.5 mg/kg q12h or 5 mg/kg q8h. Treatment of patients in the heavier weight classes should not exceed 1.5 gram/day.
When amikacin is indicated in newborns (see WARNINGS box), it is recommended that a loading dose of 10 mg/kg be administered initially to be followed with 7.5 mg/kg every 12 hours.
The usual duration of treatment is 7 to 10 days. It is desirable to limit the duration of treatment to short term whenever feasible.
The total daily dose by all routes of administration should not exceed 15 mg/kg/day. In difficult and complicated infections where treatment beyond 10 days is considered, the use of amikacin should be reevaluated. If continued, amikacin serum levels, and renal, auditory, and vestibular functions should be monitored. At the recommended dosage level, uncomplicated infections due to amikacin-sensitive organisms should respond in 24 to 48 hours. If definite clinical response does not occur within 3 to 5 days, therapy should be stopped and the antibiotic susceptibility pattern of the invading organism should be rechecked. Failure of the infection to respond may be due to resistance of the organism or to the presence of septic foci requiring surgical drainage. When amikacin is indicated in uncomplicated urinary tract infections, a dose of 250 mg twice daily may be used.
DOSAGE GUIDELINES ADULTS AND CHILDREN WITH NORMAL RENAL FUNCTION Patient Weight Dosage
lbskg 7.5 mg/kg 5 mg/kg q12h OR q8h 99 45 337.5 mg 225 mg 110 50 375 mg 250 mg 121 55 412.5 mg 275 mg 132 60 450 mg 300 mg 143 65 487.5 mg 325 mg 154 70 525 mg 350 mg 165 75 562.5 mg 375 mg 176 80 600 mg 400 mg 187 85 637.5 mg 425 mg 198 90 675 mg 450 mg 209 95 712.5 mg 475 mg 220 100 750 mg 500 mg Intramuscular Administration for Patients with Impaired Renal Function
Whenever possible, serum amikacin concentrations should be monitored by appropriate assay procedures. Doses may be adjusted in patients with impaired renal function either by administering normal doses at prolonged intervals or by administering reduced doses at a fixed interval.
Both methods are based on the patient's creatinine clearance or serum creatinine values since these have been found to correlate with aminoglycoside half-lives in patients with diminished renal function. These dosage schedules must be used in conjunction with careful clinical and laboratory observations of the patient and should be modified as necessary. Neither method should be used when dialysis is being performed.
Normal Dosage at Prolonged Intervals
If the creatinine clearance rate is not available and the patient's condition is stable, a dosage interval in hours for the normal dose can be calculated by multiplying the patient's serum creatinine by 9, e.g., if the serum creatinine concentration is 2 mg/100 mL, the recommended single dose (7.5 mg/kg) should be administered every 18 hours.
Reduced Dosage at Fixed Time Intervals
When renal function is impaired and it is desirable to administer amikacin at a fixed time interval, dosage must be reduced. In these patients, serum amikacin concentrations should be measured to assure accurate administration of amikacin and to avoid concentrations above 35 mcg/mL. If serum assay determinations are not available and the patient's condition is stable, serum creatinine and creatinine clearance values are the most readily available indicators of the degree of renal impairment to use as a guide for dosage.
First, initiate therapy by administering a normal dose, 7.5 mg/kg, as a loading dose. This loading dose is the same as the normally recommended dose which would be calculated for a patient with a normal renal function as described above.
To determine the size of maintenance doses administered every 12 hours, the loading dose should be reduced in proportion to the reduction in the patient's creatinine clearance rate:
observed CC in mL/min
Maintenance Dose Every 12 Hours = ---------------------------x calculate loading dose in mg
normal CC in mL/min(CC-creatinine clearance rate) An alternate rough guide for determining reduced dosage at 12-hour intervals (for patients whose steady state serum creatinine values are known) is to divide the normally recommended dose by the patient's serum creatinine.
The above dosage schedules are not intended to be rigid recommendations but are provided as guides to dosage when the measurement of amikacin serum levels is not feasible.
Intravenous Administration
The individual dose, the total daily dose, and the total cumulative dose of amikacin sulfate are identical to the dose recommended for intramuscular administration. The solution for intravenous use is prepared by adding the contents of a 500 mg vial to 100 or 200 mL of sterile diluent such as 0.9% sodium chloride injection or 5% dextrose injection or any of the compatible solutions listed below.
The solution is administered to adults over a 30 to 60 minute period. The total daily dose should not exceed 15 mg/kg/day and may be divided into either 2 or 3 equally-divided doses at equally-divided intervals.
In pediatric patients the amount of fluid used will depend on the amount of amikacin ordered for the patient. It should be a sufficient amount to infuse the Amikacin Sulfate Injection over a 30 to 60 minute period. Infants should receive a 1 to 2 hour infusion.
Amikacin should not be physically premixed with other drugs but should be administered separately according to the recommended dose and route.
Stability in IV Fluids
Amikacin sulfate is stable for 24 hours at room temperature at concentrations of 0.25 and 5 mg/mL in the following solutions:
5% Dextrose Injection
5% Dextrose and 0.2% Sodium Chloride Injection
5% Dextrose and 0.45% Sodium Chloride Injection
0.9% Sodium Chloride Injection
Lactated Ringer's Injection
Normosol® M in 5% Dextrose Injection (or Plasma-Lyte 56 Injection in 5% Dextrose in Water)
Normosol® R in 5% Dextrose Injection (or Plasma-Lyte 148 Injection in 5% Dextrose in Water)
In the above solutions with Amikacin Sulfate Injection concentrations of 0.25 and 5 mg/mL, solutions aged for 60 days at 4°C and then stored at 25°C had utility times of 24 hours.
At the same concentrations, solutions frozen and aged for 30 days at - 15°C, thawed, and stored at 25°C had utility times of 24 hours. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Aminoglycosides administered by any of the above routes should not be physically premixed with other drugs but should be administered separately. Because of the potential toxicity of aminoglycosides, "fixed dosage" recommendations which are not based upon body weight are not advised. Rather, it is essential to calculate the dosage to fit the needs of each patient.
-
HOW SUPPLIED
Amikacin Sulfate Injection USP, 250 mg/mL is supplied as a clear colorless to light straw colored solution which requires no refrigeration. At times the solution may become a very pale yellow; this does not indicate a decrease in potency.
Amikacin Sulfate Injection USP, 250 mg/mL
Strength NDC Packing configuration 500 mg per 2 mL 67184-0569-2 10 Single-Dose Vials (NDC: 67184-0569-1) in a carton 1 gram per 4 mL 67184-0570-2 10 Single-Dose Vials (NDC: 67184-0570-1) in a carton Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
Manufactured by:
Qilu Pharmaceutical Co., Ltd., Jinan, 250104, ChinaRevised: 09/2023
- Principal display panel - 500 mg/2 mL - label
- Principal display panel - 500 mg/2 mL - carton
- Principal display panel -1 g/4 mL - label
- Principal display panel -1 g/4 mL - carton
-
INGREDIENTS AND APPEARANCE
AMIKACIN SULFATE
amikacin sulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67184-0569 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIKACIN SULFATE (UNII: N6M33094FD) (AMIKACIN - UNII:84319SGC3C) AMIKACIN 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SULFURIC ACID (UNII: O40UQP6WCF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67184-0569-2 10 in 1 CARTON 03/19/2024 1 NDC: 67184-0569-1 2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218146 03/19/2024 AMIKACIN SULFATE
amikacin sulfate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67184-0570 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIKACIN SULFATE (UNII: N6M33094FD) (AMIKACIN - UNII:84319SGC3C) AMIKACIN 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SULFURIC ACID (UNII: O40UQP6WCF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67184-0570-2 10 in 1 CARTON 03/19/2024 1 NDC: 67184-0570-1 4 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218146 03/19/2024 Labeler - Qilu Pharmaceutical Co., Ltd. (653878256) Establishment Name Address ID/FEI Business Operations Qilu Pharmaceutical Co., Ltd. (Biological Industrial Park) 544532200 manufacture(67184-0569, 67184-0570) , analysis(67184-0569, 67184-0570)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.