MAGNESIUM CHLORIDE injection
Magnesium Chloride by
Drug Labeling and Warnings
Magnesium Chloride by is a Prescription medication manufactured, distributed, or labeled by Mylan Institutional LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Magnesium Chloride Injection is a sterile solution of Magnesium Chloride Hexahydrate in Water for Injection q.s. Each mL contains Magnesium Chloride Hexahydrate 200 mg, Sodium Chloride 9 mg, Benzyl Alcohol 1% as a preservative, Water for Injection, q.s. pH adjusted with Hydrochloric Acid and/or Sodium Hydroxide. Total osmolarity equivalent to 2.951 mOsm/mL.
Contains 1.97 mEq of Mg++ and Cl- per mL.
The structural formula is MgCl26H2O
- ACTIONS
- INDICATIONS
- CONTRAINDICATIONS
- WARNING
-
PRECAUTIONS
The usual precautions for parenteral administration should be observed. Administer with caution if flushing and sweating occurs. A preparation of a calcium salt should be readily available for intravenous injection to counteract potential serious signs of magnesium intoxication. As long as deep tendon reflexes are active it is probable that the patient will not develop respiratory paralysis. Respiration and blood pressure should be carefully observed during and after administration of Magnesium Chloride Injection.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with magnesium chloride. It is also not known whether magnesium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Magnesium Chloride should be given to a pregnant woman only if clearly needed.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
- USUAL DOSAGE RANGE
-
HOW SUPPLIED
Magnesium Chloride Injection 200 mg/mL (20% w/v).
NDC: 67457-134-50
50 mL Multiple-Dose Vial. Individually boxed.Store at 20° to 25°C (68° to 77°F). [See USP Controlled
Room Temperature.]Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103Manufactured by:
Mylan Institutional
Galway, Ireland1084L100
Revised: 5/2018
MI:MAGNIJ:R3 -
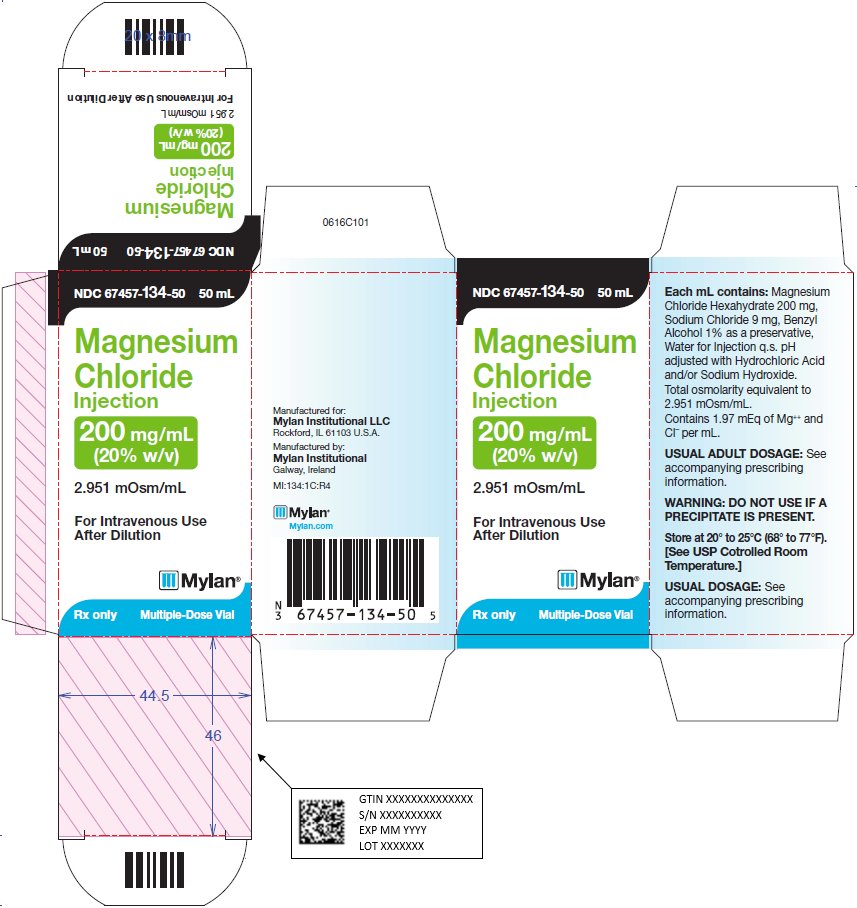
PRINCIPAL DISPLAY PANEL - 200 mg/mL
NDC: 67457-134-50 50 mL
Magnesium
Chloride
Injection200 mg/mL
(20% w/v)2.951 mOsm/mL
For Intravenous Use After
DilutionRx only Multiple-Dose Vial
Each mL contains: Magnesium
Chloride Hexahydrate 200 mg,
Sodium Chloride 9 mg, Benzyl
Alcohol 1% as a preservative,
Water for Injection q.s. pH adjusted
with Hydrochloric Acid and/or
Sodium Hydroxide.Total osmolarity equivalent to
2.951 mOsm/mL.Contains 1.97 mEq of Mg++ and CI¯
per mL.WARNING: DO NOT USE IF A
PRECIPITATE IS PRESENT.Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]Usual Dosage: See accompanying
prescribing information.Manufactured for:
Mylan Institutional LLC
Rockford, IL 61103 U.S.A.Manufactured by:
Mylan Institutional
Galway, IrelandMI:134:1C:R4
Mylan.com
-
INGREDIENTS AND APPEARANCE
MAGNESIUM CHLORIDE
magnesium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67457-134 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CHLORIDE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 10 mg in 1 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67457-134-50 1 in 1 CARTON 03/14/2013 1 50 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 03/14/2013 Labeler - Mylan Institutional LLC (790384502)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.